Extended Data Figure 1. Validation of stains to detect myeloid cells.
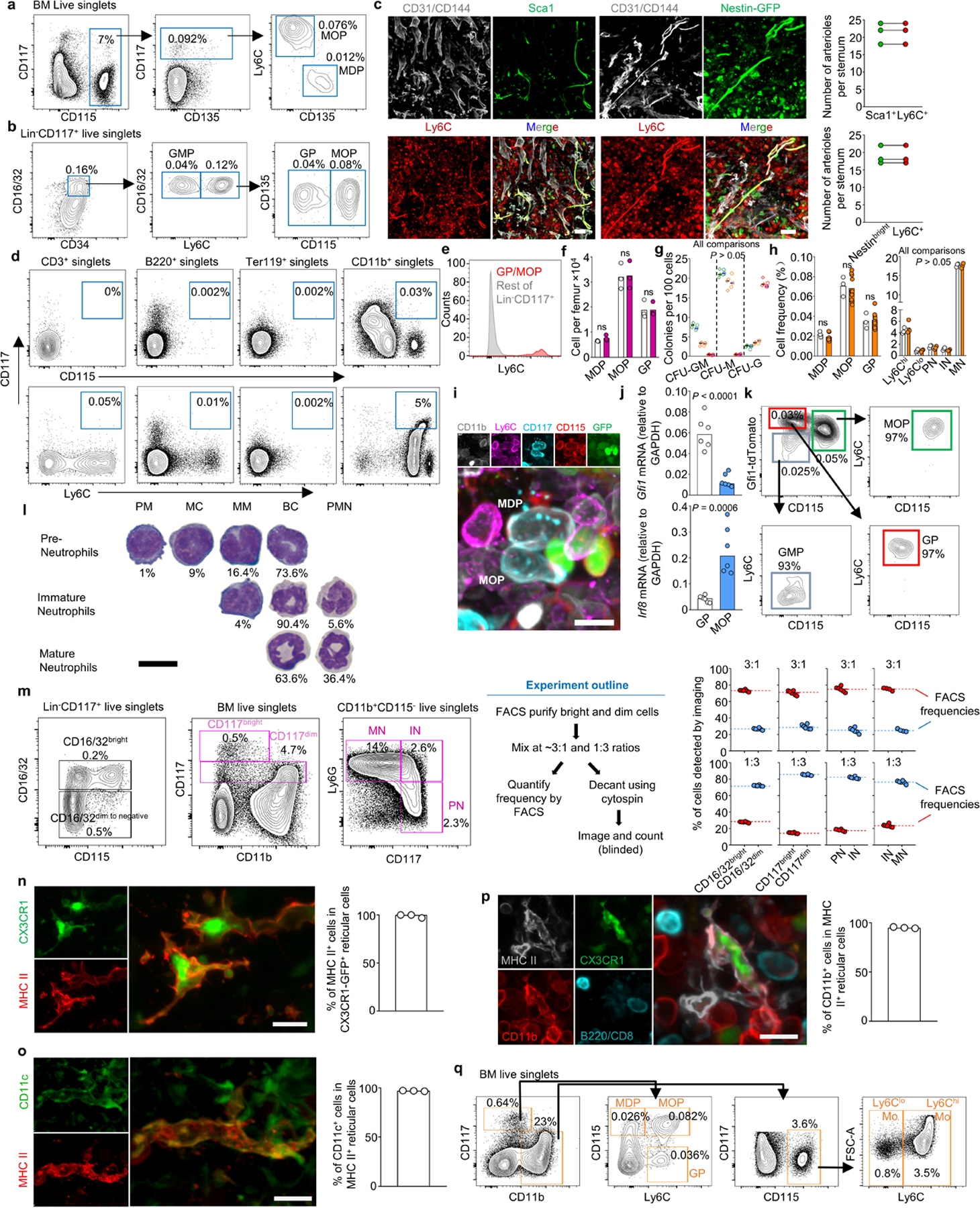
a, FACS plots showing the gating strategy to identify MDP and MOP (as described in reference19). b, Gating strategy to identify GMP, GP, and MOP (as described in reference16). The Lineage panel contains antibodies against Ly6G, CD11b, Ter119, B220 and CD3. c, Images showing that Ly6C labels arterioles detected as either CD31+CD144+Sca1bright in wild-type mice or CD31+CD144+Nestinbright structures in Nestin-GFP mice; the histograms show quantifications demonstrating that all Sca1+ arterioles or Nestin-GFPbright arterioles are also Ly6C+. Scale bars = 50 μm. d, e, FACS plots (d) showing that only the CD11b+ gate contains CD117+CD115+ or CD117+Ly6C+ cells and histogram (e) showing that GP and MOP are the only Ly6C+ cells in the Lin-CD117+ gate. Together these data indicate that CD11b alone can be used to replace the Lineage panel to exclude contamination of mature cells when detecting MDP, MOP and GP. f, g, Cell numbers per femur (f) and colony forming activity (g, green: MDP, orange: MOP, red: GP; n = total 3 mice in two experiments) of the indicated progenitors using previously described strategies16,19 (diamonds) or the one described in Fig1.g (circles). h, Frequency of total BM cells for each of the indicated populations in sternum when detected by FACS (white) or imaging (orange). i. Representative image showing that CD11b-CD117+CD115+Ly6C- MDP and CD11b-CD117+CD115+Ly6C+ MOP are GFP+ in Cx3cr1-gfp mice. Scale bar = 10 μm. j, qPCR showing Gfi1 and Irf8 expression (relative to Gapdh) in FACS-purified GP or MOP; n = total 6 mice in three experiments. k, FACS analyses in Gfi1-tdTomato mice showing differential tdTomato expression in GP and MOP in Gfi1-Tdtomato mice. l, Quantification of promyelocytes (PM), myelocytes (MC), metamyelocytes (MM), banded cells (BC) and polymorphonucleated neutrophils (PMN) in cytospin preparations of FACS-purified Pre-Neutrophils, Immature Neutrophils and Mature Neutrophils. n = total 2 mice. Scale bar = 10 μm. m, The stains require discrimination of CD16/32, CD117, and Ly6G bright and dim cells. The panels show the gating strategy, experimental design, and quantification of frequencies of decanted CD16/32 and CD117 bright and dim cells or IN, PN, and MN when compared to frequencies obtained by FACS prior cytospin. Each dot represents one image field from two experiments. n, o, Dendritic cells can be imaged as reticulated CX3CR1-GFP+ or CX3CR1-GFP+MHCII+ cells in Cx3cr1-gfp reporter mice23,24. The images and histograms show that all reticulated GFP+ cells were also MHCII+ and CD11c+ indicating that MHCII and cell shape are sufficient to unambiguously identify DC and distinguish them from macrophages that are CXCR1-GFP-CD11c- cells24; n = total 3 mice. Scale bar = 10 μm. p, Image and histogram showing that CX3CR1-GFP+MHCII+ dendritic cells are conventional dendritic cells as they are CD11b+ but do not express B220 or CD8. Scale bar = 10 μm. n = total 3 mice q, FACS gating strategy for isolation and imaging of the indicated cells. Dendritic cells are detected as MHCII+ reticulated cells in imaging analyses. In all bar graphs one dot corresponds to one mouse. Statistical differences were calculated using two-tailed Student’s T tests and p values are shown. ns = not significant.
