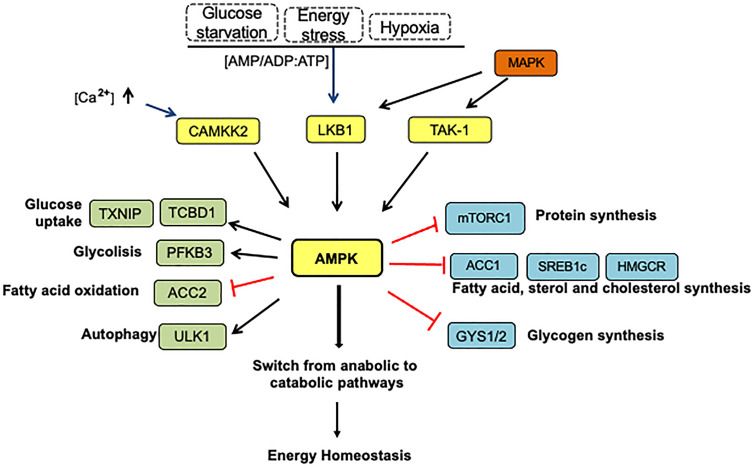
Figure 4.
The AMPK pathway activation and energy homeostasis. Under energy stress, AMPK is phosphorylated at Thr 172 by LKB1 in response to variations in AMP: ADP/ATP ratios. Other upstream kinases such as calmodulin-dependent protein kinase kinase 2 (CAMKK2) activated by intracellular calcium and transforming growth factor-β-activated kinase (TAK1) represent alternative AMPK activation forms. In this context, AMPK-activated can repress anabolic processes and increase catabolism to restore energy balance. AMPK suppresses the ATP-consuming anabolic pathways by direct phosphorylation and inhibition of several proteins: mTORC1, acetyl-CoA carboxylase (ACC1), SREBP (sterol response coactivator), HMGCoA reductase (HMGCR), which play critical roles in protein, fatty acid, sterol, and cholesterol synthesis, respectively. AMPK prevents glycogen storage by inhibitory phosphorylation of the glycogen synthases (GYS1 and GYS2). In addition, AMPK also stimulates the catabolic pathways to produce ATP by several mechanisms. First, increasing glucose utilization by phosphorylation and inactivation of domain family member 1 (TBC1D1) and thioredoxin-interacting protein (TXNIP), which control the translocation of glucose transporters GLUT4 and GLUT1 to the plasmatic membrane, respectively. Second, AMPK increases glucose flux along the glycolytic pathway by PFKFB3 (6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3) phosphorylation, which affects the PFK1 activity, a rate-limiting enzyme in glycolysis. AMPK indirectly stimulates fatty acids transport into the mitochondria by ACC2 inhibition, in turn promoting fatty oxidation. On the other hand, AMPK induces autophagy directly by ULK1 phosphorylation, a kinase essential for autophagy, and indirectly by mTORC1 inactivation. The arrows indicate: →, activation signals; ┴, inhibition signals.