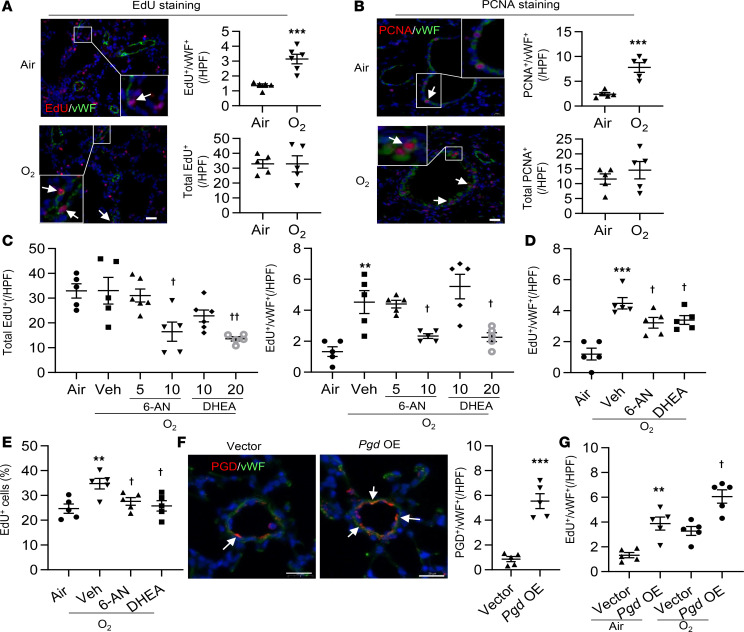
Figure 6. The PPP enhances lung EC proliferation in mice exposed to hyperoxia as neonates.
C57BL/6J neonatal mice (<12 hours old) were exposed to air or hyperoxia (95% O2) for 3 days and were then allowed for recover in room air until P14. (A) EdU was i.p. injected at 50 mg/kg daily for 3 days before sacrificing. Lung tissues were utilized for EdU staining, along with costaining with vWF. Scale bar: 20 μm. n = 5 per group. (B) Double immunofluorescence was conducted to determine the abundance of PCNA in vWF+ cells in mouse lungs. Scale bar: 20 μm. n = 5 per group. (C) 6-AN (5 and 10 mg/kg, i.p.) or DHEA (10 and 20 mg/kg, i.p.) were administered daily in mice from P9 to P13. (D) 6-AN (10 mg/kg, i.p.) or DHEA (20 mg/kg, i.p.) were administered daily in mice from P12 to P13. (C and D) Lung tissues were utilized for double immunofluorescence of EdU incorporation and vWF. Numbers of EdU+ and vWF+ cells were counted in 3 randomly selected high-power fields (HPF) for each sample. n = 5 per group. (E) EdU incorporation was measured by flow cytometry in LMVECs isolated from hyperoxia-exposed mice treated with 6-AN (10 mg/kg) or DHEA (20 mg/kg) between P9 and P13. n = 5 per group. (F) Nanoparticles mixed with plasmid DNA expressing pgd or empty vector under the control of human CDH5 promoter was administered into normoxia-exposed mice via a retro-orbital injection at P9. At P14, immunofluorescence was performed to detect colocalization of PGD and vWF in mouse lungs. Pgd OE, pgd overexpression. Scale bar: 20 μm. n = 5 per group. (G) Immunofluorescence of EdU incorporation and vWF was performed in hyperoxia-exposed mice injected with nanoparticles mixed with plasmid DNA expressing pgd or empty vector under the control of human CDH5 promoter. Numbers of EdU+/vWF+ cells were counted in 3 randomly selected high-power fields (HPF) for each sample. n = 5 per group. Data are expressed as mean ± SEM. **P < 0.01, ***P < 0.001 versus air (A–E), vector (F), or air/vector (G); †P < 0.05, ††P < 0.01 versus hyperoxia/vehicle (C–E) or hyperoxia/vector (G) using 1-tailed t test (A, B, and F) or ANOVA followed by Tukey-Kramer test (C–E, and G).