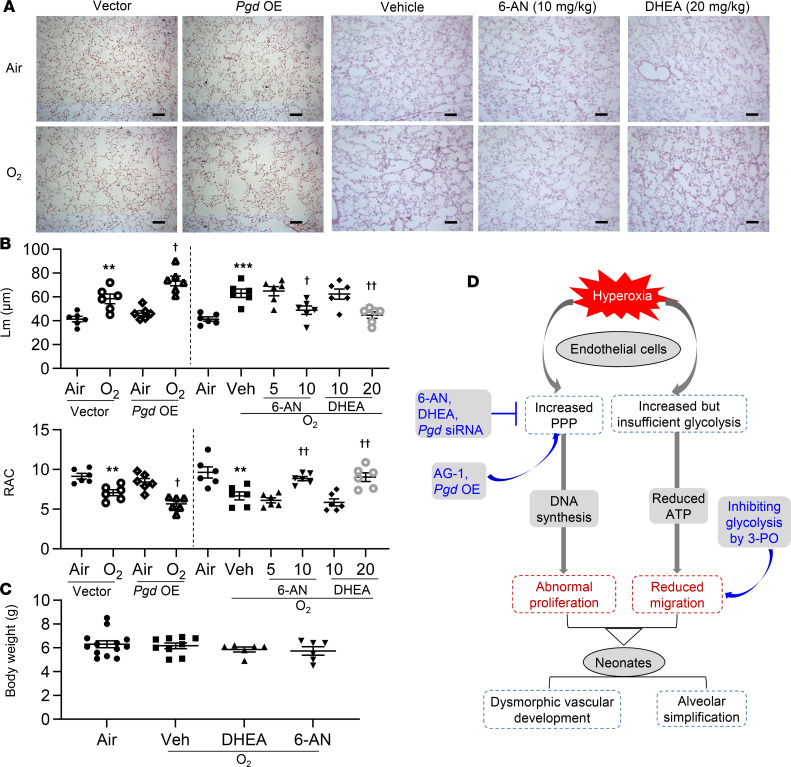
Figure 7. Endothelial pgd overexpression augments, whereas blocking the PPP attenuates, alveolar simplification in neonatal mice exposed to hyperoxia.
C57BL/6J neonatal mice (<12 hours old) were exposed to air or hyperoxia (95% O2) for 3 days and were then allowed for recover in room air until P14. At P9, mixtures of nanoparticles and plasmid DNA expressing pgd or empty vector under the control of human CDH5 promoter were administered into mice via a retro-orbital injection. 6-AN (5 and 10 mg/kg, i.p.) or DHEA (10 and 20 mg/kg, i.p.) were administered daily in mice from P9 to P13. (A) H&E staining was performed to assess lung morphology in mouse lungs. Pgd OE, pgd overexpression. Scale bar: 100 μm. (B) Mean linear intercept (Lm) and radical alveolar count (RAC) were calculated in mouse lungs. n = 6 per group. (C) Body weight was calculated after 6-AN or DHEA administration in neonatal mice exposed to hyperoxia. n = 6 per group. (D) Schematic showing that hyperoxic exposure increased the PPP and glycolysis in lung ECs. Hyperoxia-induced increase in the PPP results in abnormal EC proliferation and subsequent dysmorphic vascular development and alveolar simplification in neonates. Data are expressed as mean ± SEM. **P < 0.01, ***P < 0.001 versus air/vector or air; †P < 0.05, ††P < 0.01 versus hyperoxia/vector or hyperoxia/vehicle using ANOVA followed by Tukey-Kramer test (A–C).