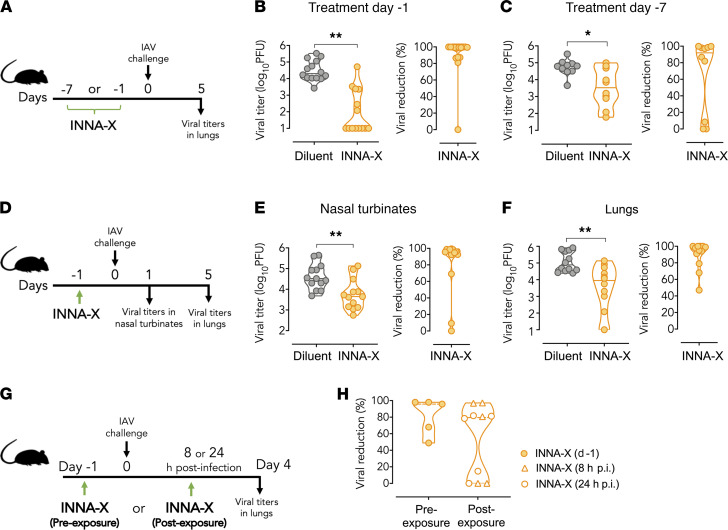
Figure 2. Inhibition of viral replication in the respiratory tract after administration of INNA-X to the URT.
(A) Mice (n = 5 or 7/group) were inoculated with 5 nmol of INNA-X prior to challenge with 500 PFU of Udorn IAV. Efficacy of treatment (B) 1 day or (C) 7 days prior to viral challenge was determined by measuring lung viral titers 5 days after infection. The percentage reduction in viral load in each mouse is shown relative to the average viral titer in similarly challenged diluent-treated mice. Results (B and C) are pooled from 2 separate experiments. (D) Mice were inoculated with 1 nmol of INNA-X and challenged 1 day later. Viral titers in (E) nasal turbinates or (F) lungs (n = 7/group) were measured at 1 or 5 days, respectively, after infection. (G) Therapeutic efficacy of treatment with INNA-X was examined by inoculating mice with INNA-X 8 or 24 hours after IAV challenge (postexposure) in comparison to treatment 1 day prior to challenge (preexposure). (H) Reduction in lung viral titers is relative to similarly infected mice treated with diluent at each time point. Statistical analysis was performed using a (B, E, and F) Mann-Whitney or (C) Welch t test. *P < 0.05, **P < 0.01.