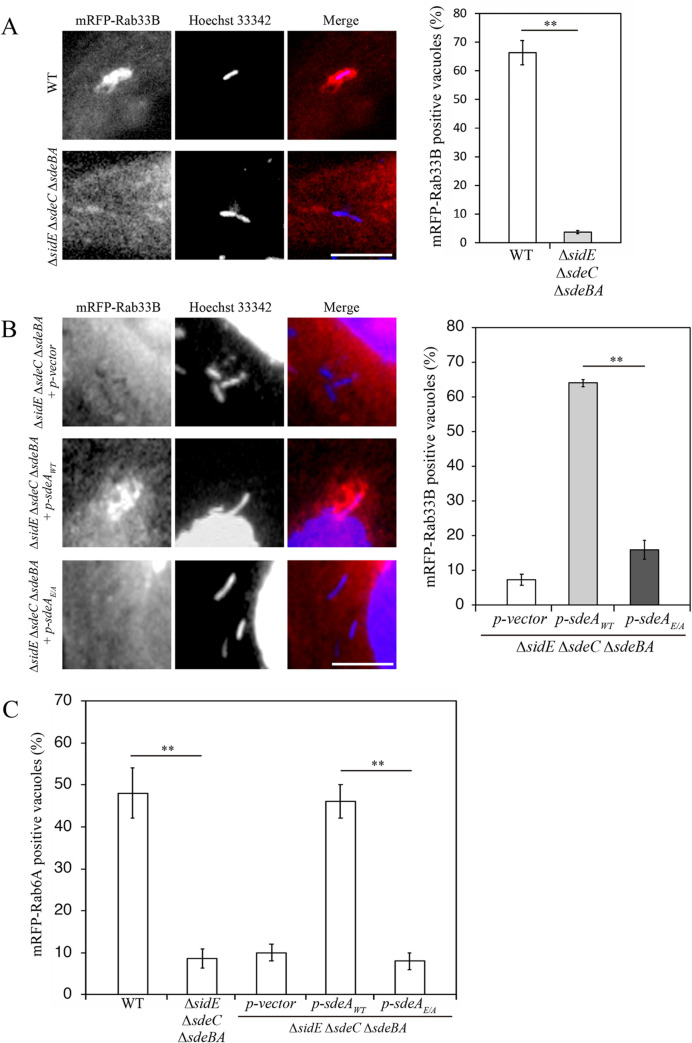
Fig 3. Phosphoribosyl-ubiquitination is necessary for LCV localization of Rab33B.
(A) HeLa-FcγRII cells transfected with a plasmid for mRFP-Rab33B for 24 h were infected with wild-type L. pneumophila (top row) or a ΔsidE ΔsdeC ΔsdeBA mutant (bottom row) for 4 h. After infection, the cells were fixed and stained with Hoechst 33342. Bar, 5 μm. The graph shows the percentage of vacuoles positive for mRFP-Rab33B. Values are the mean ± SD (n = 3, 100 vacuoles were scored in each experiment). **P < 0.01 (Student’s t test). (B) HeLa-FcγRII cells transfected with a plasmid for mRFP-Rab33B for 24 h were infected with a ΔsidE ΔsdeC ΔsdeBA L. pneumophila mutant complemented with a vector (top row), SdeA wild-type (middle row), or SdeA (2EA) (bottom row) for 4 h. After infection, the cells were fixed and stained with Hoechst 33342. Bar, 5 μm. The graph shows the percentage of vacuoles positive for mRFP-Rab33B. Values are the mean ± SD (n = 3, 100 vacuoles were scored in each experiment). **P < 0.01 (Tukey’s test). (C) HeLa-FcγRII cells transfected with a plasmid for mRFP-Rab6A for 24 h were infected with wild-type L. pneumophila, ΔsidE ΔsdeC ΔsdeBA L. pneumophila mutant, or ΔsidE ΔsdeC ΔsdeBA mutant complemented with a vector, SdeA wild-type, or SdeA (2EA) for 4 h. After infection, the cells were fixed and stained with Hoechst 33342. Bar, 5 μm. The graph shows the percentage of vacuoles positive for mRFP-Rab6A. Values are the mean ± SD (n = 3, 50 vacuoles were scored in each experiment). **P < 0.01 (Tukey’s test).