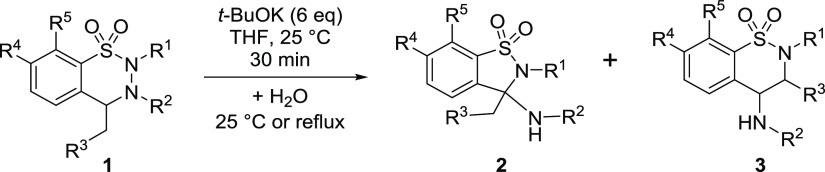
Table 2. Study on the Substituent Effect in the Reaction of Compounds 1 Carried out with 6 equiv of t-BuOK.
| entrya | 1–3 | R1 | R2 | R3 | R4 | R5 | 2:3 ratiob | yield (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | a | Me | C(O)Me | H | Cl | Cl | 0.09:1.00 | 84c (2a+3a) |
| 2 | b | Me | C(O)Me | Me | Cl | Cl | 1.00:0.88d | 54c (2b+3b) |
| 3 | c | Et | C(O)Me | H | Cl | Cl | 0.13:1.00 | 90c (2c+3c) |
| 4 | d | Bn | C(O)Me | H | Cl | Cl | 0.25:1.00 | 62c (2d+3d) |
| 5 | f | Me | C(O)Pr | H | Cl | Cl | 0.18:1.00 | 86c (2f+3f) |
| 6 | i | Me | Me | H | Cl | Cl | 1:0 | 85c (2i) |
| 7 | l | Me | C(O)Me | H | OMe | Cl | 1:0 | 74e (2l) |
| 8 | n | Me | C(O)Me | H | H | OMe | 1:0 | 35e (2n) |
| 9 | o | Me | C(O)Me | H | H | H | 1:0 | 59e (2o) |
Reagents and reaction conditions: substrate (0.6 mmol), t-BuOK (3.6 mmol), THF (3 mL), 30 min, then quenching with H2O.
The product ratio of the isolated mixtures was determined by 1H NMR.
The reaction was carried out at 25 °C.
dr = 1.00:0.16.
The reaction was carried out at reflux temperature.