Abstract
By late January 2020, the 2019 novel coronavirus (SARS-CoV-2) had reached Europe and most European countries had registered cases by March 1. However, the spread of the virus has been uneven in both prevalence and speed of propagation. We analyse the association of social, economic, and demographic factors in the initial spread of the coronavirus disease COVID-19 across 23 European countries between March 1 and April 30, 2020. Diagnosed COVID-19 cases from Johns Hopkins University and data from the European Social Survey and other sources were used to estimate bivariate associations between cumulative reported case numbers at ten-day intervals and nine social, demographic, and economic variables. To avoid overfitting, we first reduce these variables to three factors by factor analysis before conducting a multiple regression analysis. We also perform a sensitivity analysis using rates and new cases between two time periods. Results showed that social and economic factors are strongly and positively associated with COVID-19 throughout the studied period, while the association with population density and cultural factors was initially low, but by April, was higher than the earlier mentioned factors. For future influenza-like pandemics, implementing strict movement restrictions from early on will be crucial to curb the spread of such diseases in economically, socially, and culturally vibrant and densely populated countries.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12546-021-09257-1.
Keywords: COVID-19, Coronavirus, Social ties, Economic development, Nursing homes, Health policy
Introduction
As of late April 2020, there were more than three million confirmed cases worldwide of the coronavirus disease COVID-19 (CSSE, 2020) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). While enquiries are still underway regarding the exact origins of the virus, the first case in humans was identified in late 2019 in Wuhan, China. Subsequent person-to-person transmission then mainly took place through respiratory droplets produced through coughs or sneezes of infected persons in close presence of other people (Huang et al. 2020; Peeri et al. 2020).
One well-established cause of the global spread of previous influenza outbreaks is airplane travel (Grais et al. 2003) and without this mode of transportation the coronavirus would not have arrived in Europe so quickly. The first cases were confirmed in Europe in late January-early February and, although international travel restrictions to and from China were quickly imposed, by late March about half of the world’s reported cases were in Europe (CSSE, 2020). Once the virus was brought to Europe, human interaction and close contact allowed the virus to spread quickly. Previous research on virus transmission, including on COVID-19, has shown social contact to be very important (Bayer & Kuhn, 2020; Bi et al. 2020; Liu et al. 2020; Mossong et al. 2008; Wallinga et al. 2006). Based on modelled estimates using both empirical (the POLYMOD survey) and synthetic data, Prem, van Zandvoort, et al. (2020) showed how altering (intergenerational) patterns of contact by reducing physical contact or shielding would lead to large reductions in the transmission of the virus. At the same time, cultural differences in Europe are well established. For instance, in Southern European countries intergenerational contact is more frequent than in the less family-oriented Western and Northern European countries, as social norms about providing support to family members and maintaining interpersonal familiar interactions are stronger there (Reher, 1998; Sánchez Rodríguez et al. 2014). In relation to COVID-19 case fatality rates, Arpino et al. (2020) recently showed it to be broadly positively associated with intergenerational co-residence and contacts at the national level across a selection of European countries. However, as conclusive interpretations could not be derived because the association did not hold at the province level in the case of Italy, the authors advocated considering confounding factors when analysing the effect of intergenerational relations.
Notwithstanding, as patterns in social mixing are embedded in socioeconomic and cultural factors and are very different across Europe, this continent remains an excellent geographical area for study of COVID-19 proliferation. The present study therefore analyses statistical associations between different indicators of social and economic ties and the reported number of confirmed cases of COVID-19 in 23 European countries between March 1 and April 30, 2020.
Data and method
Data on the number of confirmed cases of COVID-19 come from the Center for Systems Science and Engineering at Johns Hopkins University (CSSE, 2020). Data on the covariates come from different sources and relate to years as close to 2020 as was possible to obtain (see notes under Table 1). Data from the European Social Survey, Eurostat, the World Bank, and the OECD were used to approximate different types of social and cultural ties, which we hypothesize to be positively associated with COVID-19: average number of household members; percentage living in a multi-generational household; proportion of people who have frequent social meetings with friends, relatives, or colleagues; and religious attendance. In addition, we also test the effect of the socioeconomic variables tertiary education and GDP per capita, as we assume that higher educated or more economically developed countries are more likely to pursue activities that require travelling (e.g., international business meetings, skiing), factors which contributed to the initial outbreak of the epidemic. Lastly, we test the effect of demographic variables: the share of the population aged 65 + , population density and per capita number of beds in nursing and residential care facilities (all expected to be positively associated with COVID-19) (Table 1).
Table 1.
Descriptive statistics of the number of reported confirmed COVID-19 cases and the covariates used in the analysis
| Country | Area | Code | Date of the first case con-firmed | Cumulative cases of COVID-19 on | Population as at 1/1/2019 | Confirmed cases on 31/03/ 100,000 people | Confirmed cases on 30/04/ 100,000 people | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 01/03 | 11/03 | 21/03 | 31/03 | 10/04 | 20/04 | 30/04 | |||||||
| Austria | West | AT | 25/02 | 14 | 246 | 2814 | 10,180 | 13,555 | 14,795 | 15,452 | 8,858,775 | 114.91 | 174.43 |
| Belgium | West | BE | 04/02 | 2 | 314 | 2815 | 12,775 | 26,667 | 39,983 | 48,519 | 11,455,519 | 111.52 | 423.54 |
| Bulgaria | East | BG | 08/03 | 0 | 7 | 163 | 399 | 635 | 929 | 1506 | 7,000,039 | 5.70 | 21.51 |
| Czechia | East | CZ | 01/03 | 3 | 91 | 995 | 3308 | 5732 | 6900 | 7682 | 10,649,800 | 31.06 | 72.13 |
| Denmark | North | DK | 27/02 | 4 | 444 | 1420 | 3039 | 6014 | 7711 | 9356 | 5,806,081 | 52.34 | 161.14 |
| Estonia | East | EE | 27/02 | 1 | 16 | 306 | 745 | 1258 | 1535 | 1689 | 1,324,820 | 56.23 | 127.49 |
| Finland | North | FI | 29/01 | 6 | 59 | 523 | 1418 | 2769 | 3868 | 4995 | 5,517,919 | 25.70 | 90.52 |
| France | West | FR | 24/01 | 130 | 2293 | 14,463 | 52,827 | 91,738 | 155,393 | 167,299 | 67,012,883 | 78.83 | 249.65 |
| Germany | West | DE | 27/01 | 130 | 1908 | 22,213 | 71,808 | 122,171 | 147,065 | 163,009 | 83,019,213 | 86.50 | 196.35 |
| Hungary | East | HU | 04/03 | 0 | 13 | 103 | 492 | 1190 | 1984 | 2775 | 9,772,756 | 5.03 | 28.40 |
| Ireland | West | IE | 29/02 | 1 | 43 | 785 | 3235 | 8089 | 15,652 | 20,612 | 4,904,240 | 65.96 | 420.29 |
| Italy | South | IT | 31/01 | 1694 | 12,462 | 53,578 | 105,792 | 147,577 | 181,228 | 205,463 | 60,359,546 | 175.27 | 340.40 |
| Lithuania | East | LT | 28/02 | 1 | 3 | 83 | 537 | 999 | 1326 | 1385 | 2,794,184 | 19.22 | 49.57 |
| Netherlands | West | NL | 27/02 | 10 | 503 | 3640 | 12,667 | 23,249 | 33,588 | 39,512 | 17,282,163 | 73.30 | 228.63 |
| Norway | North | NO | 26/02 | 19 | 598 | 2118 | 4641 | 6314 | 7156 | 7738 | 5,328,212 | 87.10 | 145.23 |
| Poland | East | PO | 04/03 | 0 | 31 | 536 | 2311 | 5955 | 9593 | 12,877 | 37,972,812 | 6.09 | 33.91 |
| Portugal | South | PT | 02/03 | 0 | 59 | 1280 | 7443 | 15,472 | 20,863 | 25,045 | 10,276,617 | 72.43 | 243.71 |
| Serbia | East | RS | 06/03 | 0 | 12 | 171 | 900 | 3105 | 6630 | 9009 | 6,963,764 | 12.92 | 129.37 |
| Slovenia | East | SI | 05/03 | 0 | 57 | 383 | 802 | 1160 | 1335 | 1429 | 2,080,908 | 38.54 | 68.67 |
| Spain | South | ES | 01/02 | 84 | 2277 | 25,374 | 95,923 | 158,273 | 200,210 | 213,435 | 46,937,060 | 204.37 | 454.73 |
| Sweden | North | SE | 31/01 | 14 | 500 | 1763 | 4435 | 9,685 | 14,777 | 21,601 | 10,230,185 | 43.35 | 211.15 |
| Switzerland | West | CH | 25/02 | 27 | 652 | 6575 | 16,605 | 24,551 | 27,944 | 29,586 | 8,544,527 | 194.33 | 346.26 |
| The UK | West | UK | 31/01 | 36 | 459 | 5067 | 25,481 | 74,605 | 125,856 | 178,771 | 66,647,112 | 38.23 | 268.24 |
| EU 23 total | 24/01 | 2176 | 23,047 | 147,168 | 437,763 | 750,763 | 1,026,321 | 1,188,745 | 490,739,135 | 89.20 | 174.43 | ||
| Country | Mean number of household members | % living in a multi-generational household | % having a frequent social meeting | % ≥ weekly religious attendance | % tertiary education | Population per km2 | % people aged 65 + | GDP per capita | Nursing/ rest home beds / 100,000 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Austria | 2.2 | 5 | 65.5 | 11.4 | 31.1 | 107.21 | 19.00 | 51,462 | 862.4 | |||||||||
| Belgium | 2.3 | 4.8 | 68.6 | 8.6 | 36.0 | 377.21 | 18.79 | 47,519 | 1,234.1 | |||||||||
| Bulgaria | 2.4 | 24.7 | 51.7 | 9.3 | 24.7 | 64.70 | 21.02 | 9273 | 30.8 | |||||||||
| Czechia | 2.4 | 7.6 | 50.8 | 5.8 | 21.6 | 137.60 | 19.42 | 23,079 | 687.5 | |||||||||
| Denmark | 2.0 | 1.2 | 71.2 | 4.3 | 33.1 | 138.07 | 19.81 | 61,350 | 816.1 | |||||||||
| Estonia | 2.2 | 8.9 | 43.5 | 3.7 | 36.5 | 30.39 | 19.63 | 23,266 | 870.7 | |||||||||
| Finland | 2.0 | 1.5 | 65.1 | 4.6 | 38.5 | 18.16 | 21.72 | 50,152 | 1,190.0 | |||||||||
| France | 2.2 | 2.0 | 67.9 | 7.6 | 33.7 | 122.34 | 20.03 | 41,464 | 981.5 | |||||||||
| Germany | 2.0 | 5.3 | 59.7 | 7.1 | 25.9 | 237.37 | 21.46 | 47,603 | 1,152.2 | |||||||||
| Hungary | 2.3 | 11.2 | 20.4 | 10.6 | 22.5 | 107.91 | 19.16 | 16,162 | 853.3 | |||||||||
| Ireland | 2.6 | 8.1 | 58.9 | 33.9 | 40.7 | 70.45 | 13.87 | 78,806 | 639.3 | |||||||||
| Italy | 2.3 | 14.2 | 60.1 | 27.4 | 17.4 | 205.45 | 22.75 | 34,483 | 415.8 | |||||||||
| Lithuania | 2.2 | 9.4 | 31.1 | 17.1 | 37.9 | 44.53 | 19.71 | 19,153 | 726.3 | |||||||||
| Netherlands | 2.2 | 2.7 | 74.5 | 9.8 | 34.8 | 511.46 | 19.20 | 53,024 | 1,379.6 | |||||||||
| Norway | 2.0 | 2.3 | 78.5 | 3.9 | 37.7 | 14.55 | 17.05 | 81,697 | 765.6 | |||||||||
| Poland | 2.8 | 16.1 | 30.9 | 46.5 | 28.2 | 124.04 | 17.52 | 15,421 | 195.3 | |||||||||
| Portugal | 2.5 | 13.1 | 77.6 | 27.8 | 23.8 | 112.24 | 21.95 | 23,408 | 555.7 | |||||||||
| Serbia | 2.9 | 21.3 | 62.1 | 11.1 | 20.6 | 79.83 | 18.35 | 7247 | 291.5 | |||||||||
| Slovenia | 2.5 | 16.1 | 53.1 | 12.6 | 29.3 | 102.64 | 19.61 | 26,124 | 1,012.4 | |||||||||
| Spain | 2.5 | 17.9 | 70.2 | 15.2 | 35.1 | 93.53 | 19.38 | 30,371 | 834.8 | |||||||||
| Sweden | 2.0 | 1.2 | 74.3 | 5.7 | 37.8 | 25.00 | 20.10 | 54,608 | 1,388.0 | |||||||||
| Switzerland | 2.2 | 2.3 | 71.6 | 8.3 | 38.6 | 215.52 | 18.62 | 82,797 | 1,174.2 | |||||||||
| The UK | 2.3 | 3.9 | 62.4 | 11.7 | 40.6 | 274.83 | 18.40 | 42,944 | 821.4 | |||||||||
Source: Confirmed COVID-19 cases: Center for Systems Science and Engineering at Johns Hopkins University (CSSE, 2020). Population on 1 January 2019 (Eurostat: https://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=demo_pjan&lang=en). Mean number of household members 2018 (Eurostat: https://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=ilc_lvph01&lang=en), % living in a multi-generational household (people who live with i) parents, ii) parents and children, iii) parents and relatives, iv) parents, children, and relatives, v) children and relatives, or vi) relatives), % having a frequent social meeting (people who socially meet with their friends, relatives, or colleagues at least once a week) and % ≥ weekly religious attendance are created from European Social Survey (ESS) Rounds 7 (2014) (Denmark), 8 (2016) (Lithuania, Portugal, Spain and Sweden) and 9 (2018) (all other countries). % tertiary education of 25–64 year-olds in 2018 (Eurostat: http://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=edat_lfse_03&lang=eng), except BGR and SRB (ESS)). Population per km2 in 2018 (World Bank: https://data.worldbank.org/indicator/EN.POP.DNST), % people aged 65 + in 2018 (World Bank: https://data.worldbank.org/indicator/SP.POP.65UP.TO.ZS) and GDP in 2018 (World Bank: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD). Available beds in nursing and residential care facilities per 100.000 inhabitants in 2017, except for Belgium (2012) and Denmark (2011) and Portugal (applied 2017 ratio of long-term care recipients between Portugal and Spain to the beds available in Spain); Eurostat (https://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=hlth_rs_bdsns&lang=en) and OECD (https://stats.oecd.org/Index.aspx?ThemeTreeId=9).
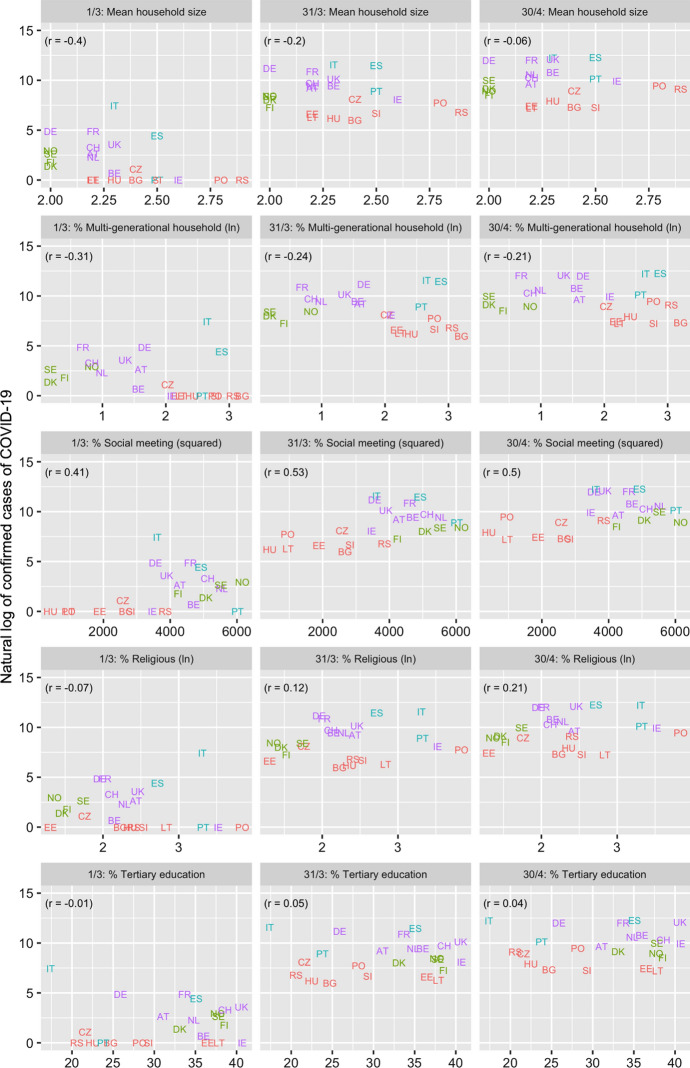
Bivariate associations between the covariates and the natural logarithm (ln) of the cumulative number of confirmed COVID-19 cases between March 1 and April 30 are analysed at 10-day intervals to ascertain whether the direction and strength of the associations changed over time. A later date was not analysed due to country-differences in the severity and timing of movement and social contact restrictions that European governments implemented during this period, thus confounding the effect of the tested social and economic tie variables. In the supplementary material file the analyses are repeated for COVID-19 cases per 100,000 population and the number of cases during each 10-day period.
Ordinary linear regression analysis was used to assess the unique association between confirmed cases of COVID-19 and the covariates. However, as covariate data could only be obtained for 23 countries, i.e., too few to test all covariates simultaneously without overfitting (Harrell Jr et al. 1984; Peduzzi et al. 1996), we first opted to reduce the number of variables by performing a factor analysis. This method not only simplifies the subsequent analysis, it also alerts us to groupings of variables that we would not otherwise have thought of, enabling us to work at a more sophisticated conceptual level (De Vaus, 2002).
The factor analysis yielded three sociodemographic-like latent factors that explained 78% of the country-variation in the selected covariates. Each latent factor is highly associated (> 0.75) with one or more covariates, and this is why Factor 1 has been labelled “socially and economically vibrant”, Factor 2 “relatively young population”, and Factor 3 “densely populated and traditional” (see Tables S1-S4 in the Supplementary file). Correlation coefficients between COVID-19 and the obtained latent factors were first calculated (Table 2) before performing the multivariate regression analyses1 to obtain the adjusted R2 of the models (Table 3) and the unstandardized coefficients, i.e., the factors’ slope (Table S5).
Table 2.
Correlation between the natural log COVID-19 in 23 European countries at six different time periods and the covariates
| Variable | March 1 | March 11 | March 21 | March 31 | April 10 | April 20 | April 30 |
|---|---|---|---|---|---|---|---|
| 1. Mean number of household members | −0.40 | −0.38 | −0.28 | −0.20 | −0.13 | −0.08 | −0.06 |
| 2. % living in a multi-generational household (ln) | −0.31 | −0.42* | −0.29 | −0.24 | −0.23 | −0.21 | −0.21 |
| 3. % having a frequent social meeting (squared) | 0.41 | 0.61** | 0.56** | 0.53** | 0.52* | 0.50* | 0.50* |
| 4. % ≥ weekly religious attendance (ln) | −0.07 | −0.10 | 0.03 | 0.12 | 0.17 | 0.20 | 0.21 |
| 5. % tertiary education | −0.01 | 0.06 | 0.05 | 0.05 | 0.05 | 0.05 | 0.04 |
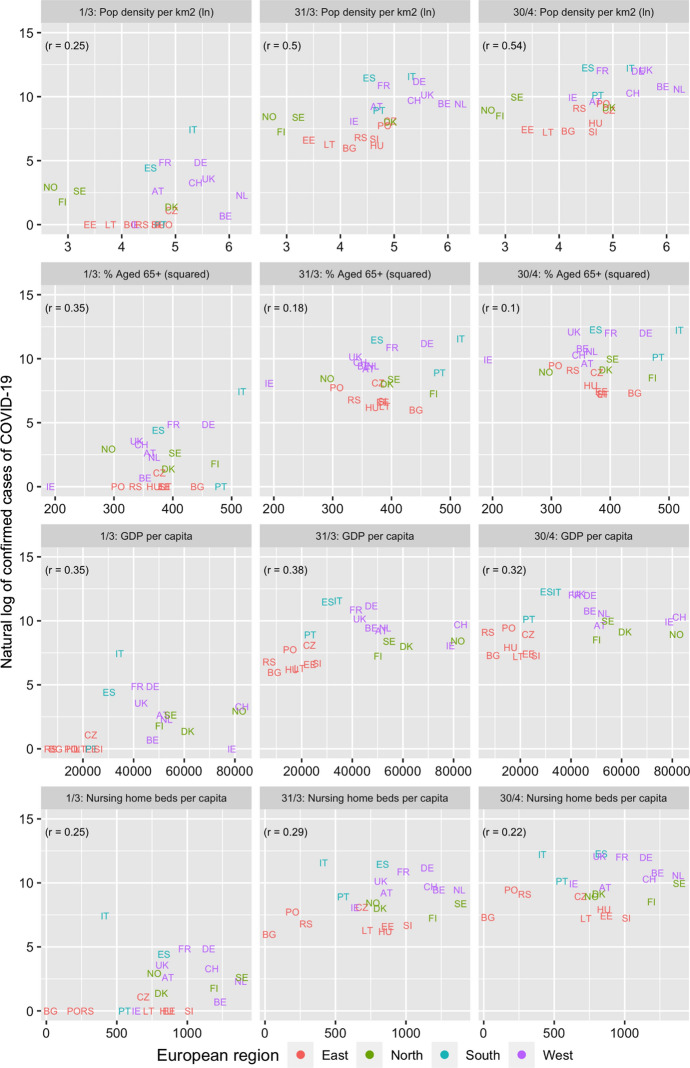
| 6. Population density (people per km2) (ln) | 0.25 | 0.37 | 0.44* | 0.50* | 0.53** | 0.54** | 0.54** |
| 7. % people aged 65 + (squared) | 0.35 | 0.22 | 0.23 | 0.18 | 0.14 | 0.10 | 0.10 |
| 8. GDP per capita | 0.35 | 0.51* | 0.44* | 0.38 | 0.35 | 0.33 | 0.32 |
| 9. Nursing and rest home beds per capita | 0.25 | 0.39 | 0.31 | 0.29 | 0.27 | 0.24 | 0.22 |
**p < 0.01, *p < 0.05
Table 3.
Correlation between the natural log of COVID-19 in 23 European countries at six different time periods and the extracted factors
| Date of cumulative cases of COVID-19 | |||||||
|---|---|---|---|---|---|---|---|
| March 1 | March 11 | March 21 | March 31 | April 10 | April 20 | April 30 | |
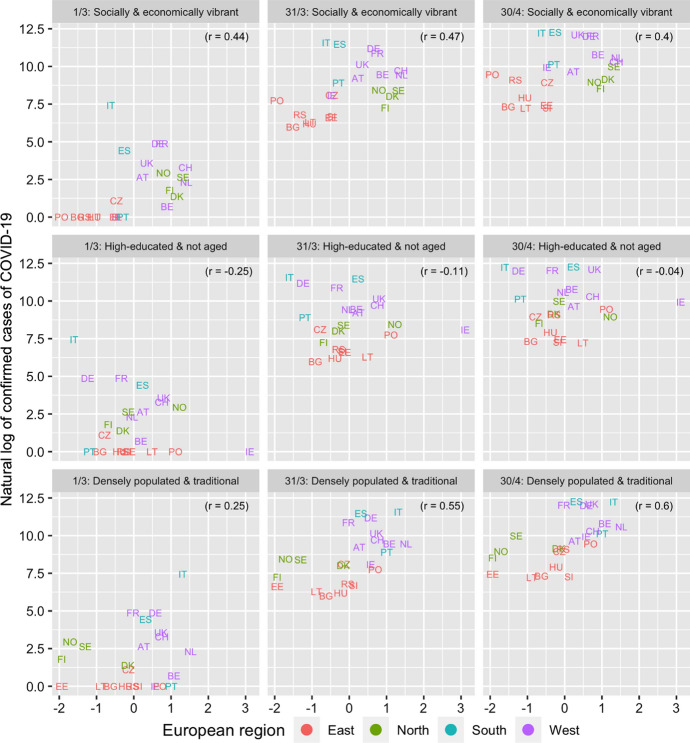
| Factor 1: Socially and economically vibrant | 0.44* | 0.61** | 0.52** | 0.47** | 0.44* | 0.41 | 0.40 |
| Factor 2: Relatively young population | −0.26 | −0.16 | −0.14 | −0.11 | −0.07 | −0.04 | −0.04 |
| Factor 3: Densely populated and traditional | 0.25 | 0.38* | 0.48** | 0.55** | 0.59** | 0.60** | 0.60** |
**p < 0.01, *p < 0.05
Results
Figure 1 and Table 2 present the association between the number of confirmed cases of COVID-19 and the different covariates for the six dates between March 1 and April 30, 2020. The highest (and significant) associations are observed for the social meeting and population density variables with the latter association becoming stronger over time. Figure 2 and Table 3 present the association between the three extracted factors and COVID-19. The “socially and economically vibrant” factor has a strong and positive association throughout the study period. The “relatively young population” factor is negatively associated (as expected) with COVID-19 but its p-values are insignificant. On the other hand, the association of the “densely populated and traditional” factor was initially low but increased with time, becoming the most important factor by the end of March. The three factors together explain close to 50% of the cross-country variation in the number of confirmed cases of COVID-19 between March 11 and April 30, compared to just 21% on March 1 (Table 4). The slope of the “socially and economically vibrant” factor was greatest on March 11 and that of the “densely populated and traditional factor” on April 20.
Fig. 1.
Association between covariates and natural log of cumulative cases of COVID-19, March 1, 31, April 30, 2020 among 23 European countries
Fig. 2.
Association between factor scores and natural log of cumulative cases of COVID-19. March 1, 31, and April 30, 2020 among 23 European countries
Table 4.
Multivariate regression analysis of social and demographic factors on cumulative cases of the ln of COVID-19 in 23 European countries on March 1, 11, 21 and 31, and April 10, 20, and 30, 2020. Unstandardized coefficients (p-value)
| Unstandardized coefficients (p value) | |||||||
|---|---|---|---|---|---|---|---|
| March 1 | March 11 | March 21 | March 31 | April 10 | April 20 | April 30 | |
| Factor 1: Socially & economically vibrant | 0.91 (0.033) | 1.30 (0.001) | 0.93 (0.004) | 0.81 (0.007) | 0.75 (0.010) | 0.70 (0.017) | 0.66 (0.022) |
| Factor 2: Relatively young population | −0.53 (0.194) | −0.35 (0.304) | −0.26 (0.377) | −0.18 (0.506) | −0.12 (0.649) | −0.07 (0.795) | −0.07 (0.803) |
| Factor 3: Densely populated & traditional | 0.53 (0.197) | 0.82 (0.022) | 0.86 (0.007) | 0.96 (0.002) | 0.99 (0.001) | 1.01 (0.001) | 1.00 (0.001) |
| R2 adjusted | 0.21 | 0.48 | 0.44 | 0.46 | 0.47 | 0.46 | 0.45 |
If we analyse the change in COVID-19 cases over 10-day periods rather than the absolute number of cumulative cases, results are virtually the same (Supplementary Table S5a). The “socially and economically vibrant” factor is strongly significant during March and the first 10 days of April, while the “densely populated and traditional factor” was significant throughout the entire study period and became the most important explanatory factor from March 21. The “relatively young population” factor showed little association in any of the models. The proportion of the country differences in change in COVID-19 explained by the three factors equalled 44–48%, dropping down to 33% during the last 10 days of April). Conversely, the factors explain much less of the country differences in the number of cases of COVID-19 per 100,000 population. The correlation with the “socially and economically vibrant” factor is above 0.4 from March 31 onwards and the same applies to the densely populated and traditional factor 10 days later (which again becomes the most important explanatory factor) (Supplementary Table S6). The explanatory power increased steadily over time from 3% (March 1) to 43% (April 30). This is consistent with the fact that towards the end of April, the countries with a high number of cases per 100,000 population included not only Italy, but also the densely populated Belgium and the Netherlands, while COVID-19 rates were (still) quite low in the sparsely populated Scandinavian and Baltic countries (Supplementary Figure S4).
Discussion
Confirmed cases of COVID-19 increased sharply across Europe during March and April of 2020. Throughout most of the studied period, Italy was worst hit by the pandemic in absolute numbers, but Spain surpassed Italy in early April, while Belgium and Ireland did so in terms of cases per 100,000 people. The question we posed is whether social, economic, and demographic factors could explain the observed differences in Europe.
Our results suggest that it is not so much how aged countries are but their (historical) level of economic development and (associated) social ties that may have led to the initial spread of the COVID-19 pandemic. While these factors continued to be important throughout the analysed period, population density and cultural factors also contributed to the subsequent diffusion of the virus.
Considering specific examples, the Netherlands, Switzerland, and Sweden all scored high on the “socially and economically vibrant” factor and saw their number of coronavirus infections quickly increase during March despite households being almost exclusively single-person or nuclear. An important component of this factor, however, is also the number of available beds in nursing and residential care facilities, in which all three countries score high. Recent studies have shown that nursing homes may be responsible for 19% to 72% of COVID-19 deaths (Comas-Herrera et al. 2020; Orange, 2020). On the other hand, Italy, which was the initial epicentre of the pandemic in Europe, scored very low on the “relatively young population” factor, but high on the “densely populated and traditional” factor. In other words, its aged population, high population density and traditional values (approximated through the proportion weekly church attendants) is likely to have contributed to their high rate of diagnosed cases. Moreover, the relative position of other traditionally catholic countries in COVID-19, including Spain, Portugal and Belgium worsened markedly between March 21 and April 10 (Fig. 2).
While the country differences in COVID-19 cases per 100,000 people across the 23 European countries could only be weakly explained by the three factors in early March, by mid-April, both the “socially and economically vibrant” and “densely populated and traditional” factors contributed significantly to the explanation of the European country differences, as the number of cases increased markedly in the most socioeconomically developed and densely populated European countries. More specifically, by analysing changes in COVID-19 cases over 10-day periods, we found that during the early stage of the epidemic (early March) social and economic ties appeared to be most important, while population density and church attendance explained more of the growth in diagnosed cases from late March until late April (the end of the analysed study period). In light of research from elsewhere, it is noteworthy to mention that the role of religious services in the spread of the coronavirus in South Korea is well-documented through field investigations that established the source of infection for many cases (Prem, Liu, et al., 2020). Likewise, regarding the initial spread in Europe, Bartscher et al. (2020) also found the coronavirus to be initially more prevalent in high social capital areas. On the other hand, the effect of a relatively young, highly educated population (Factor 2) was not associated with the number of registered COVID-19 infections. We know that during the first wave of the pandemic few asymptomatic and/or young people were tested (Kohns Vasconcelos et al. 2021; Surkova et al. 2020), so this could explain why the association was not positive. Conversely, as results also showed that multigenerational households was the most important variable in the “socially and economically vibrant” factor and highly positive with COVID-19, it suggests that it is not the proportion of elderly per se that leads to higher rates of COVID-19, but the level of social interaction (as well as the number people in residential care homes). In this context and supported by evidence from studies that analysed intergenerational co-residence (Esteve et al. 2020) and contact patterns (Prem, van Zandvoort, et al., 2020), tailored public health responses, in particular shielding policy for the elderly, are recommended during the early stage of corona-type of virus epidemics to not only reduce their impact on the health of individuals and public health care systems, but also on the economy (see also Prem, Liu, et al., 2020; Prem, van Zandvoort, et al., 2020; Davies et al. 2020).
Some limitations of our study should be mentioned. First, we did not consider country differences in (the timing of) government (and individual) responses to the COVID-19 pandemic. Governments have differed in the timing of the implementation of measures such as cancelling public events, closing day care centres, schools and universities, social distancing, or partial or total lockdowns (Flaxman et al. 2020). This implies that the effect of social and demographic factors on COVID-19 cases may be confounded by these measures in those countries that were quickest at adopting them and had already past their peak of daily additional cases of COVID-19 (e.g., Italy). Given the estimated average latency period between becoming infected by the coronavirus and reported COVID-19, we think that only the last two data points may be affected by this.
Other factors are also likely to be responsible for the spread of the coronavirus in Europe. A French and Austrian ski resort was responsible for initial infections in the UK and other Northern European countries (Flaxman et al. 2020; Hruby, 2020), but tertiary education and GDP variables are likely to capture the influence of winter holidays or international travel on country differences in COVID-19 during the studied period. Smoking is another variable associated with the proliferation of COVID-19 due to its social function (Paul et al. 2010) and because smokers are more likely to touch their face and mouth and have chronic health conditions (GBD, 2015 Tobacco Collaborators 2017; Science Media Centre, 2020). However, we think that smoking is a more important factor to consider in individual or small-area studies, as analysis showed smoking rates to be higher in (mainly Eastern European) countries where the coronavirus was late in getting a hold.
Another issue of concern is country differences in testing for COVID-19. Some countries only test people admitted to hospitals or ramped up the testing program much later during the first outbreak than other countries. This implies that particularly some of the earlier data points will be an underestimate of the real prevalence of COVID-19 as it mainly pertains to symptomatic people (Farge & Revill, 2020; Kohns Vasconcelos et al. 2021; Wikipedia, 2020). Apart from unknown symptomacy, the data did not contain information on the place where the coronavirus was contracted. Such data has only been used in (family) case cluster studies (e.g. Chan et al. 2020; Danis et al. 2020; Fong et al. 2020). In the context of our study, of particular interest would have been being able to distinguish between the proportion of infections that took place outside the home (more likely among children and people employed) and those within the household (more probable among older people and those living in multigenerational or overcrowded households). As we analysed different periods of the first wave, such information could have provided us with more insight into the importance of particular variables for the different settings of infection.
A recurrent problem of any national-level analysis that uses aggregate data is that any association found might not necessarily reflect associations that are observed at the individual level, a shortcoming known as the “ecological fallacy”. That said, we did not obtain results contradictory to what we expected.
Finally, data from the ESS is not available for all European countries, implying that different results may be obtained if data for other countries become available.
To conclude, the main take away message for public health policy is that, while disentangling the effect of a variegated number of social, cultural, economic, and demographic factors on the diffusion of the COVID-19 epidemic is a difficult task, the level of importance of specific determinants in spreading the virus is likely to change over time. In a European setting we found that factors associated with the level of economic development and social ties were particularly important initially, while population density and cultural factors were likely to have facilitated further spread of the virus once it took hold. However, as Chinazzi et al. (2020) showed, the implementation of international travel restrictions would not be enough to curb the initial spread of a virus, as disease transmissibility also needs to be reduced through public health interventions and behavioural changes. Examples of the efficacy of border management policies and stringent public health interventions are Taiwan and New Zealand. Although New Zealand was helped by its isolation, Taiwan managed even better to limit the number of reported infections, despite its close proximity to the Chinese mainland, through its existing disease and outbreak surveillance systems and effective means of face mask distribution and promotion. Both countries also had strict border management policies, quarantining rules, and secure facilities for incoming travellers in place and developed contact tracing (Summers et al. 2020). Conversely, European island nations, particularly Ireland and Iceland, were clearly much less successful in dealing with the first wave of the pandemic as infection rates were one of the highest in the world (CSSE, 2020). Based on our results we therefore recommend for future outbreaks of coronavirus-like epidemics when no vaccine is yet available, quick implementation of travel restrictions and very strict measures of social distancing. This should be especially done in densely populated countries with strong international economic and social ties, in order to minimise the proliferation of cases during the secondary transmission phase that occurs within households and nursing homes. A recommendation for future research is to perform a European analysis at the sub-national level, given the unequal distribution of COVID-19 within countries (e.g., the north vs. the south of Italy).
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
RM had the original idea for the paper. JS performed the analysis and wrote the first draft. Both authors contributed to the final draft.
Funding
Funding was received from the Spanish Ministry of Science, Innovation and Universities (RYC-2013-14851; CSO2017-89721-R; RTI2018-096730-B-I00), the European Research Council (ERC-2019-CoG-864616) and from the Catalan Government under the CERCA Program.
Data availability and code
Details of the sources are provides in the article, but the created data file and codes used to perform the analysis can be obtained from the corresponding author upon request.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Ethical approval
We only used publically available secondary data, so no ethics compliance required.
Footnotes
As the rotated factors are orthogonal and thus not correlated, each covariate’s standardized coefficient in the multivariate regression analysis is the same as the bivariate Pearson’s correlation coefficient.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arpino B, Bordone V, & Pasqualini M (2020) Are intergenerational relationships responsible for more COVID-19 cases? A cautionary tale of available empirical evidence. Proceedings of the National Academy of Sciences. 10.1073/pnas.2008581117
- Bartscher AK, Seitz S, Slotwinski M, Siegloch S, & Wehrhöfer N (2020) Social capital and the spread of Covid-19: Insights from European countries. CESifo Working Paper, No. 8346 [DOI] [PMC free article] [PubMed]
- Bayer C, & Kuhn M (2020) Intergenerational ties and case fatality rates: A cross-country analysis. IZA Discussion Paper, 13114https://voxeu.org/article/intergenerational-ties-and-case-fatality-rates.
- Bi Q, Wu Y, Mei S, Ye C, Zou X, Zhang Z, et al. Epidemiology and transmission of COVID-19 in Shenzhen China: Analysis of 391 cases and 1,286 of their close contacts. medrxiv. 2020 doi: 10.1101/2020.03.03.20028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comas-Herrera A, Zalakain J, Litwin C, Hsu AT, Lane N, & Fernandez-Plotka JL (2020) Mortality associated with COVID-19 outbreaks in care homes: early international evidence. https://ltccovid.org/
- CSSE (2020). Novel Coronavirus (COVID-19) Cases. https://github.com/CSSEGISandData/COVID-19.
- Chan JF-W, Yuan S, Kok K-H, To KK-W, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400. doi: 10.1126/science.aba9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danis K, Epaulard O, Bénet T, Gaymard A, Campoy S, Botelho-Nevers E, et al. Cluster of coronavirus disease 2019 (COVID-19) in the French Alps, February 2020. Clinical Infectious Diseases. 2020;71(15):825–832. doi: 10.1093/cid/ciaa424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, N. G., Kucharski, A. J., Eggo, R. M., Gimma, A., Edmunds, W. J., Jombart, T., et al. (2020). Effects of non-pharmaceutical interventions on COVID-19 cases, deaths, and demand for hospital services in the UK: a modelling study. The Lancet Public Health. 10.1016/S2468-2667(20)30133-X [DOI] [PMC free article] [PubMed]
- De Vaus D. Surveys in social science. 5. Routledge; 2002. [Google Scholar]
- Esteve A, Permanyer I, Boertien D, Vaupel JW. National age and coresidence patterns shape COVID-19 vulnerability. Proceedings of the National Academy of Sciences. 2020 doi: 10.1073/pnas.2008764117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farge E, & Revill J (2020) ‘Test, test, test’: WHO chief’s coronavirus message to world. Reuters, March 16. https://www.reuters.com/article/us-healthcare-coronavirus-who/test-test-test-who-chiefs-coronavirus-message-to-world-idUSKBN2132S4.
- Flaxman S, Mishra S, Gandy A, Unwin HJT, Coupland H, Mellan TA, et al. (2020) Estimating the number of infections and the impact of non-pharmaceutical interventions on COVID-19 in 11 European countries. https://arxiv.org/abs/2004.11342.
- Fong M, Cowling B, Leung G, Wu P. Letter to the editor: COVID-19 cases among school-aged children and school-based measures in Hong Kong, July 2020. Eurosurveillance. 2020;25(37):2001671. doi: 10.2807/1560-7917.ES.2020.25.37.2001671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2015 Tobacco Collaborators Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: a systematic analysis from the Global Burden of Disease Study 2015. The Lancet. 2017;389(10082):1885–1906. doi: 10.1016/S0140-6736(17)30819-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grais RF, Ellis JH, Glass GE. Assessing the impact of airline travel on the geographic spread of pandemic influenza. European Journal of Epidemiology. 2003;18(11):1065–1072. doi: 10.1023/A:1026140019146. [DOI] [PubMed] [Google Scholar]
- Harrell FE, Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Statistics in medicine. 1984;3(2):143–152. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- Hruby D (2020) How an Austrian ski resort helped coronavirus spread across Europe. https://edition.cnn.com/2020/03/24/europe/austria-ski-resort-ischgl-coronavirus-intl/index.html.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohns Vasconcelos, M., Renk, H., Popielska, J., Nyirenda Nyang’wa, M., Burokiene, S., Gkentzi, D. et al. (2021). SARS-CoV-2 testing and infection control strategies in European paediatric emergency departments during the first wave of the pandemic. European Journal of Pediatrics, 180, 1299–1305. [DOI] [PMC free article] [PubMed]
- Liu Y, Eggo RM, Kucharski AJ. Secondary attack rate and superspreading events for SARS-CoV-2. The Lancet. 2020;395(10227):e47. doi: 10.1016/S0140-6736(20)30462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossong J, Hens N, Jit M, Beutels P, Auranen K, Mikolajczyk R, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS medicine. 2008;5(3):e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange, R. (2020). Anger in Sweden as elderly pay price for coronavirus strategy. The Guardian, 19 April. https://www.theguardian.com/world/2020/apr/19/anger-in-sweden-as-elderly-pay-price-for-coronavirus-strategy.
- Paul CL, Ross S, Bryant J, Hill W, Bonevski B, Keevy N. The social context of smoking: a qualitative study comparing smokers of high versus low socioeconomic position. BMC Public Health. 2010;10(1):211. doi: 10.1186/1471-2458-10-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. Journal of Clinical epidemiology. 1996;49(12):1373–1379. doi: 10.1016/S0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- Peeri, N.C., Shrestha, N., Rahman, M.S., Zaki, R., Tan, Z., Bibi, S., et al. (2020) The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? International Journal of Epidemiology, 49(3), 717–726. 10.1093/ije/dyaa033. [DOI] [PMC free article] [PubMed]
- Prem, K., Liu, Y., Russell, T.W., Kucharski, A.J., Eggo, R.M., Davies, N., et al. (2020). The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. The Lancet Public Health. Lancet Public Health, 5, e261–70. 10.1016/S2468-2667(20)30073-6. [DOI] [PMC free article] [PubMed]
- Prem K, van Zandvoort K, Klepac P, Eggo RM, Davies NG, Cook AR, et al. Projecting contact matrices in 177 geographical regions: an update and comparison with empirical data for the COVID-19 era. medrxiv. 2020 doi: 10.1101/2020.07.22.20159772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reher DS. Family ties in Western Europe: persistent contrasts. Population and Development Review. 1998;24(2):203–234. doi: 10.2307/2807972. [DOI] [Google Scholar]
- Sánchez Rodríguez MM, Gierveld JDJ, Buz J. Loneliness and the exchange of social support among older adults in Spain and the Netherlands. Ageing and Society. 2014;34(2):330–354. doi: 10.1017/S0144686X12000839. [DOI] [Google Scholar]
- Science Media Centre (2020). Expert reaction to questions about smoking and COVID-19. https://www.sciencemediacentre.org/expert-reaction-to-questions-about-smoking-and-covid-19/.
- Summers, D. J., Cheng, D. H. Y., Lin, P. H. H., Barnard, D. L. T., Kvalsvig, D. A., Wilson, P. N., et al. (2020). Potential lessons from the Taiwan and New Zealand health responses to the COVID-19 pandemic. The Lancet Regional Health - Western Pacific, 100044. [DOI] [PMC free article] [PubMed]
- Surkova, E., Nikolayevskyy, V., & Drobniewski, F. (2020). False-positive COVID-19 results: hidden problems and costs. The Lancet Respiratory Medicine, 8(12), 1167–1168. 10.1016/S2213-2600(20)30453-7. [DOI] [PMC free article] [PubMed]
- Wallinga J, Teunis P, Kretzschmar M. Using data on social contacts to estimate age-specific transmission parameters for respiratory-spread infectious agents. American Journal of Epidemiology. 2006;164(10):936–944. doi: 10.1093/aje/kwj317. [DOI] [PubMed] [Google Scholar]
- Wikipedia (2020). COVID-19 testing. https://en.wikipedia.org/wiki/COVID-19_testing.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Details of the sources are provides in the article, but the created data file and codes used to perform the analysis can be obtained from the corresponding author upon request.