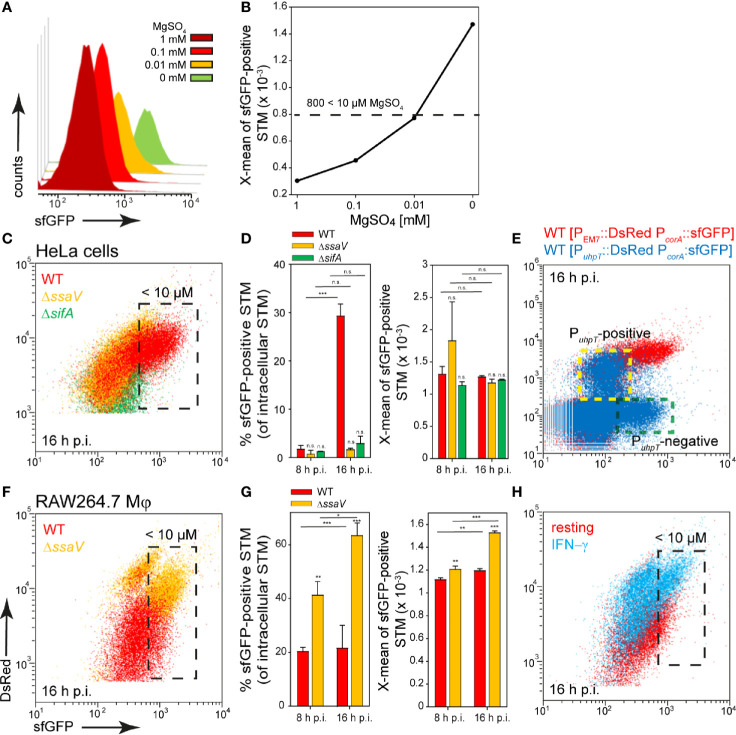
Figure 3.
A dual fluorescence reporter for measuring the intracellular magnesium availability for STM in HeLa cells or RAW264.7 macrophages. STM harboring p5078 for constitutive expression of DsRed, and sfGFP under control of PcorA was cultured in in PCN minimal medium with various amounts of MgSO4. STM WT [p5078] was grown o/n in PCN (25), pH 7.4, diluted 1:31 in fresh PCN (1), pH 7.4 with various concentrations of MgSO4 as indicated and subcultured for 3.5 h. Samples were collected after 3.5 h (A) and X-means of sfGFP intensity of PcorA-induced bacteria was determined by FC (B). sfGFP intensities above 800 RFI indicate MgSO4 concentrations lower than 10 µM. sfGFP intensities of PcorA-positive bacteria of a representative experiment are shown. The values for the induction of the magnesium reporter were derived from at least three independent experiments. Host cells were infected with STM WT (red), ΔssaV (orange), and ΔsifA (green) strains as indicated, each containing the magnesium reporter p5078 at MOI of 5. HeLa cells (C, D) or RAW264.7 macrophages (F, G) were lysed at 8 h or 16 h p.i., released STM were fixed, and subjected to FC to quantify PcorA::sfGFP intensities for at least 50,000 DsRed-positive STM per condition. Representative quantification of population size and X-mean sfGFP intensities of PcorA-positive population for STM WT, ΔssaV and ΔsifA in HeLa cells (D), or in RAW264.7 macrophages (G) at 8 h and 16 h p.i. Mean values and standard deviations of PcorA-positive bacterial populations from triplicates of a representative experiment from three biological replicates are shown. Statistical analyses were performed by one-way ANOVA for STM WT compared to mutant strains, or between time points, and are expressed as: n.s., not significant; *p < 0.05; **p < 0.01; ***p < 0.001. (E) HeLa cells were infected at MOI 5 with STM WT harboring p5189 with DsRed under control of PuhpT and PcorA::sfGFP (blue). For comparison and gating of populations, HeLa cells were infected with STM WT harboring p5078 for constitutive expression of DsRed and PcorA::sfGFP (red). Host cells were lysed 16 h p.i., released STM were fixed, and subjected to FC for quantification of PcorA-positive bacteria and induction of PuhpT. Data for STM WT [p5078] and WT [p5489] of a representative experiment from three biological replicates are shown. The cytosolic, PuhpT-induced and SCV-bound, PuhpT-negative subpopulations are indicated by yellow and green frames, respectively. (H) RAW264.7 macrophages were cultured in medium without (red) or with 5 ng x ml-1 IFN-γ (blue) for 24 h, and subsequently infected with STM WT [p5078] at MOI 5. Host cells were lysed at 16 h p.i., released STM were fixed, and subjected to FC to quantify PcorA::sfGFP intensities of at least 50,000 DsRed-positive STM. Data for STM WT [p5078] in resting RAW264.7 (red), or activated RAW264.7 (blue) of a representative experiment from three biological replicates are shown.