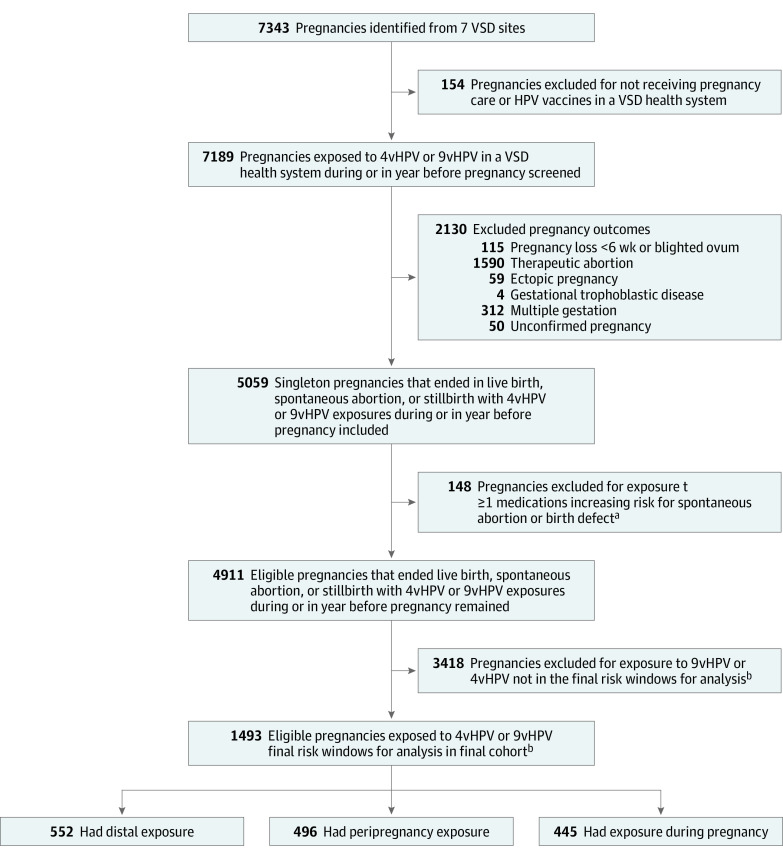
Figure 2. Flowchart of Pregnancies and Final Cohort Across 7 Vaccine Safety Datalink (VSD) Sites.
aMedications that were excluded if dispensed 6 months before last menstrual period (LMP) through the end of pregnancy were prostaglandin analogs, vitamin A analogs, selected immunosuppressants, selected anticonvulsants, amiodarone hydrochloride, warfarin sodium, and lithium carbonate.
bThe final exposure windows for analysis were as follows: distal exposure consisted of 9-valent human papillomavirus vaccine (9vHPV) or quadrivalent human papillomavirus vaccine (4vHPV) administered from 22 to 16 weeks before LMP, peripregnancy exposure consisted of 9vHPV administered from 42 days before LMP until LMP, and during-pregnancy exposure consisted of 9vHPV administered from LMP to 19 completed weeks’ gestation. For pregnancies in the distal exposure, 103 (18.7%) received 4vHPV and 449 (81.3%) received 9vHPV.