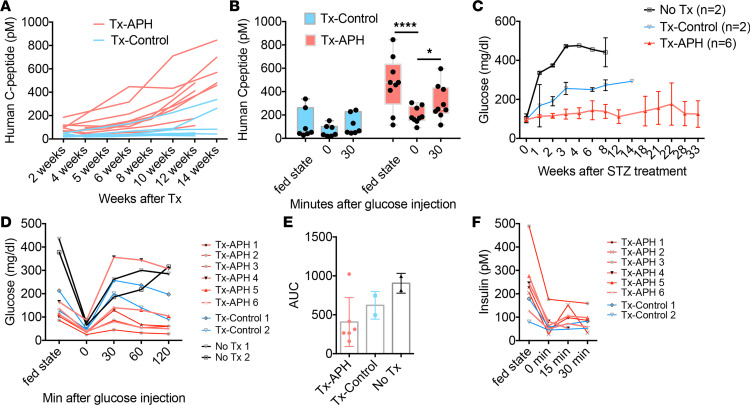
Figure 7. Mice transplanted with cells pretreated with APH secrete high human C-peptide and are protected from induced diabetes.
(A) Human C-peptide serum concentration in mice at different time points after transplantation with cells treated with APH (APH) and control cells (MEL1) at fed state. (B) Human C-peptide serum concentration in mice at 12–14 weeks after transplantation with APH cells (APH) and control cells (MEL1) at fed state, fasting, and 30 minutes after glucose injection. Two-way ANOVA *P < 0.05; ****P < 0.0001. (C) Blood glucose levels of STZ-treated mice without transplantation (No Tx) (P = 2), transplanted with control cells (Tx-Control) (n = 2), and with APH-treated cells (Tx-APH) (n = 6). Blood glucose levels of Tx-Control and No Tx mice were monitored until persistent hyperglycemia required euthanasia according to animal protocol. (D) Glucose tolerance test of STZ-treated mice in fed state, fasting state, and 15–120 minutes after glucose injection. Intraperitoneal glucose tolerance test (IPGTT) was performed on day 14 after STZ treatment. (E) Area under the curve of glucose tolerance test was calculated to compare among Tx-Control, Tx-APH, and No Tx. (F) Serum human insulin concentrations of STZ-treated mice transplanted with APH-treated cells at fed state, fasting, and 30 minutes after glucose injection.