Figure 3.
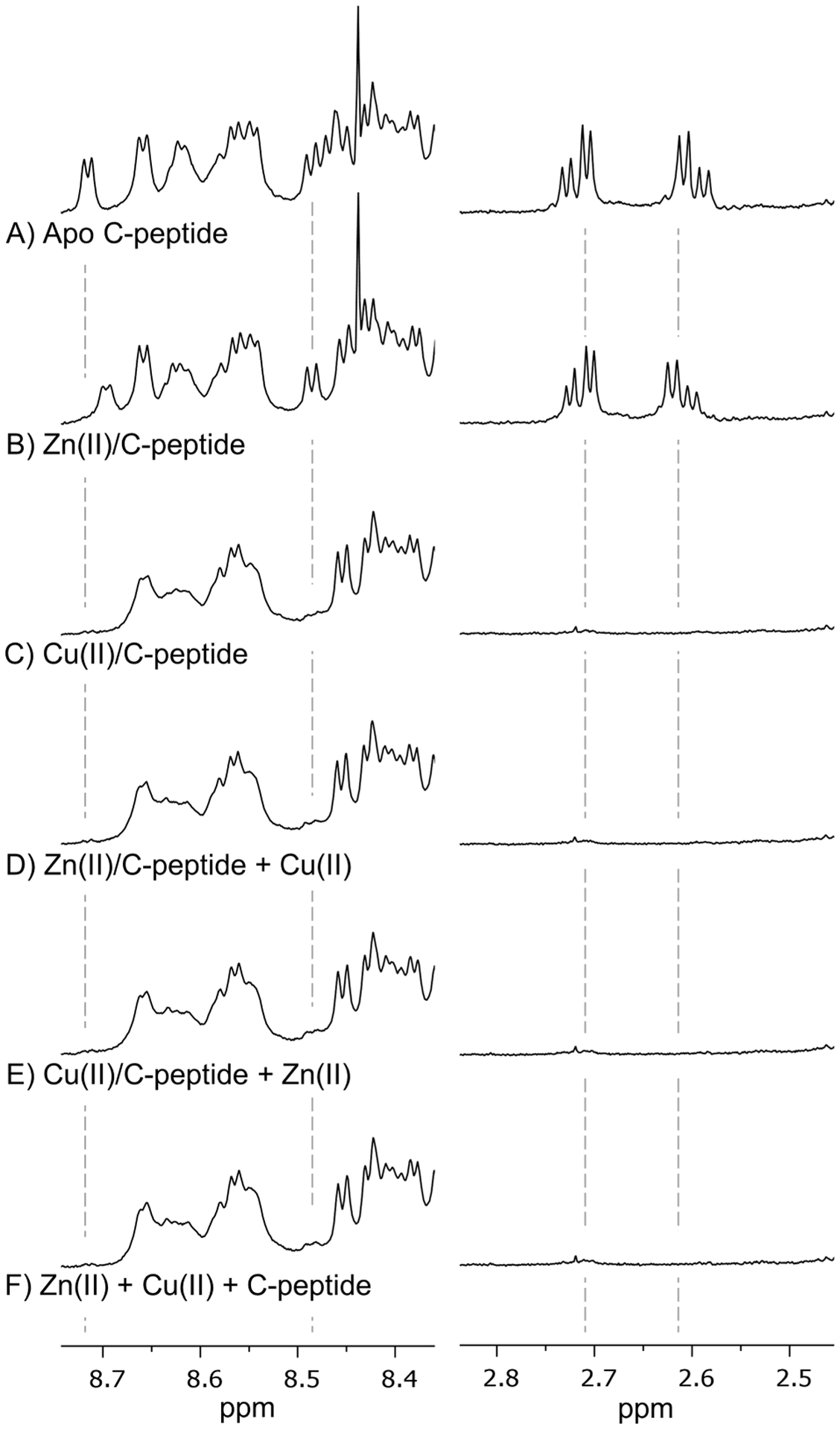
Highlighted regions of the 1H NMR spectra of (A) apo C-peptide, (B) Zn(II)/C-peptide, (C) Cu(II)/C-peptide, (D) equimolar Cu(II) added to Zn(II)/C-peptide, (E) equimolar Zn(II) added to Cu(II)/C-peptide, and (F) Cu(II) and Zn(II) added to apo C-peptide simultaneously. Full spectra are shown in Figure S1. The addition of Zn(II) induces shifts in proton resonances for CHβ protons on D4 (2.6 ppm) and backbone amide protons from D4 (8.45 ppm) and E3 (8.7 ppm), while Cu(II) obliterates proton resonances within these regions. The spectra of Cu(II) competition with Zn(II) (D–F) result in spectra that resemble Cu(II)/C-peptide and not Zn(II)/C-peptide, indicating that Cu(II) displaces Zn(II) for binding C-peptide. Solutions were prepared at 1.5 mM peptide in 95:5 (v/v) H2O/D2O with 10 mM Tris-d11 at pH 7.4, and spectra were collected at 800 MHz and 10 °C.
