Fig. 3 |. Pathological complete response rates stratified by ER status and treatment arm in clinical trials of neoadjuvant HER2-targeted therapy for HER2+ breast cancer.
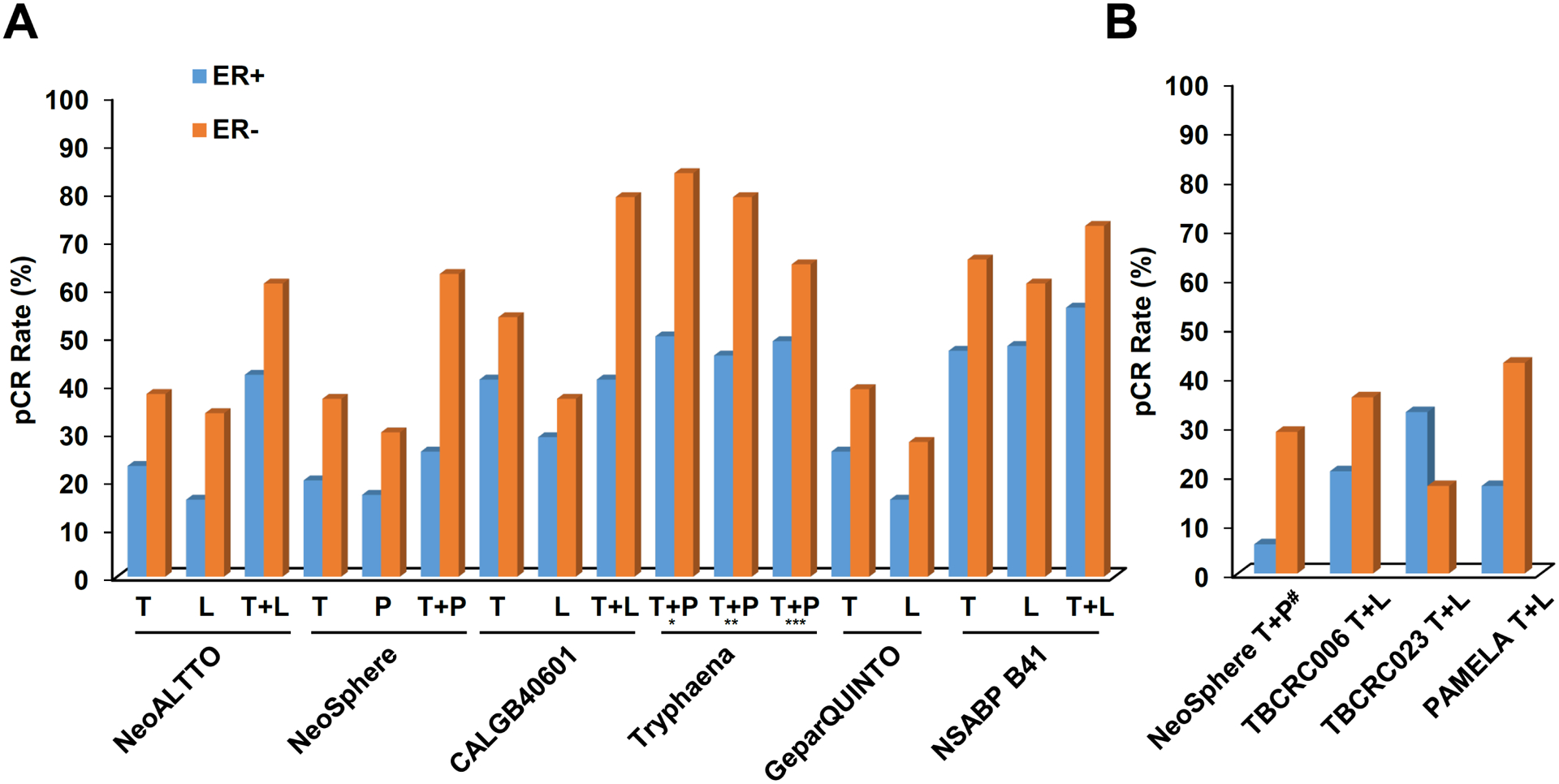
a | Pathological complete response (pCR) rates observed with HER2-targeted therapy in combination with chemotherapy. b | pCR rates observed with HER2-targeted therapy, plus endocrine therapy for the oestrogen receptor-positive (ER+) patient subgroups, without chemotherapy. For some trials, ER status actually reflects the hormone receptor status (that is, positive or negative for ER and/or progesterone receptor expression). L, lapatinib; P, pertuzumab; T, trastuzumab. aDocetaxel and carboplatin plus T + P. b5-fluorouracil, epirubicin and cyclophosphamide plus T + P followed by docetaxel plus T + P. c5-fluorouracil, epirubicin and cyclophosphamide followed by docetaxel plus T + P, dChemotherapy-free arm
