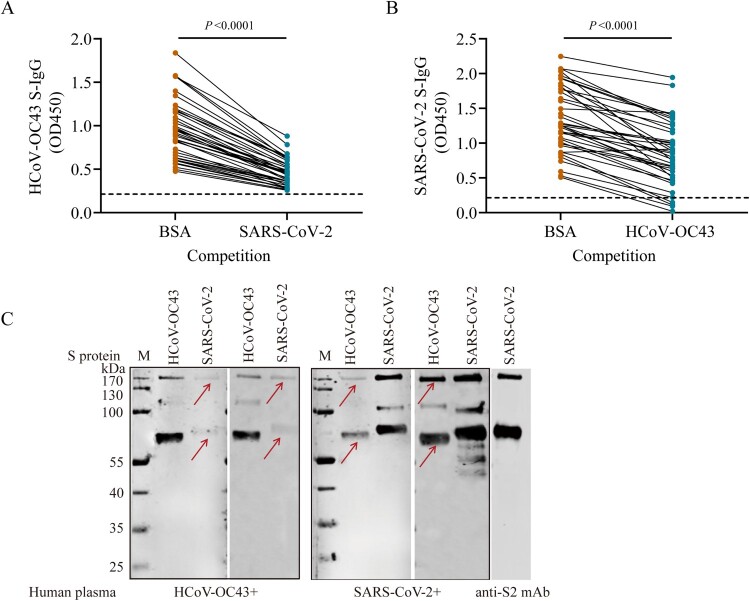
Figure 2.
Two-way antigenic cross-reactivities between SARS-CoV-2 and HCoV-OC43. (A) HCoV-OC43 S-IgG levels in plasma samples taken from COVID-19 patients (n = 50) after competition by 0.5% bovine serum albumin (BSA) or SARS-CoV-2 S protein, respectively. (B) SARS-CoV-2 S-IgG levels in plasma samples taken from COVID-19 patients (n = 50) after competition using 0.5% BSA or HCoV-OC43 S protein, respectively. (C) Western blot analysis to determine the cross-reactivity between S proteins of SARS-CoV-2 and HCoV-OC43. Two human plasma samples positive for HCoV-OC43 S-IgG and two human plasma samples positive for SARS-CoV-2 S-IgG were used to probe the S proteins of SARS-CoV-2 and HCoV-OC43, respectively. Plasma samples were diluted at 1:400 and S protein of HCoV-OC43 and SARS-CoV-2 were loaded at 250 ng/well, respectively. A monoclonal antibody against SARS-CoV-2 S2 subunit (Anti-S2 mAb) was used as positive control. The red arrows indicate ectodomain and S2 subunit of S protein, respectively. Two-tailed Wilcoxon matched-pairs signed-rank test was used for antibody level comparison.