Figure 5.
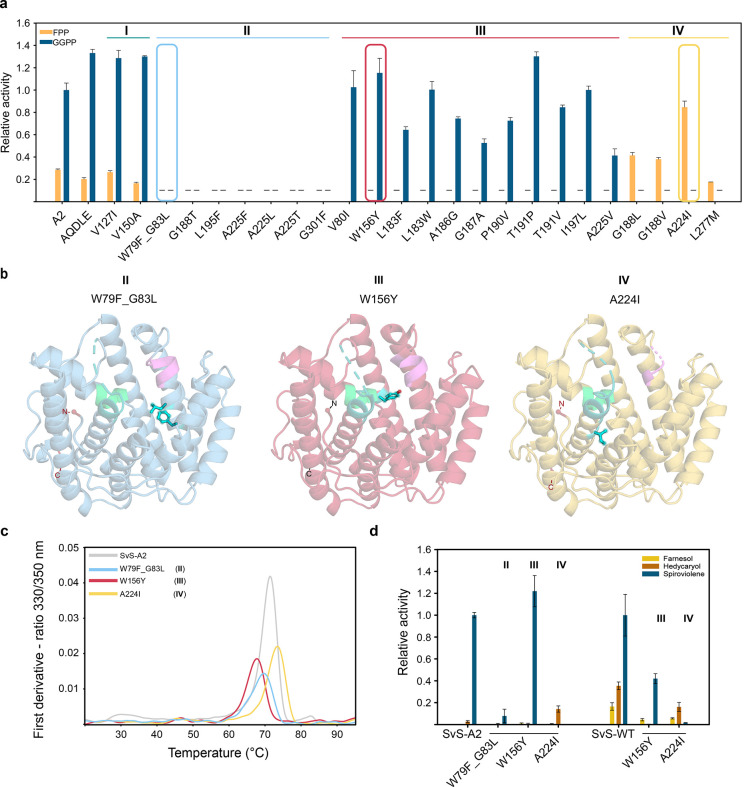
Rational enzyme engineering of ancestral terpene cyclase SvS-A2. (a) Activity of variant library based on SvS-A2 as scaffold measured by Malachite Green assay using single substrates. Variants are classified as inactive (I), promiscuous (II), GGPP-specific (III), or FPP-specific (IV). Activities are given relative to SvS-A2 GGPP activity (defined as 1.0). Variants with values below the sensitivity threshold are represented with a dash. Error bars are standard deviations from triplicates. Selected representative variants for each group are enclosed by colored boxes. (b) Crystal structures of the three representative variants from (a) with the mutated residues shown as cyan sticks and balls. The DDxx(x)D motif is shown in violet, NSE motif in cyan/teal, effector motif in light green. (c) Thermal stability of representative variants of SvS-A2. Melting temperatures were determined by nano-DSF as the maximum of the derivative of the 330/350 nm ratio (technical triplicates, one representative trace is shown). (d) Product formation by representative variants assessed by GC-FID. Triplicates using ca. 400 nM of SvS-A2 variants and 2 μM of SvS-WT variants were incubated with a mix of both 60 μM FPP and GGPP (3 h at 30 °C) in vitro (different enzyme concentration used due to different protein stability). Product formation was quantified relative to an internal standard. Activities are given relative to SvS-A2 GGPP activity (for SvS-A2 variants) and relative to SvS-WT GGPP activity (for SvS-WT variants, each defined as 1.0, respectively).