Figure 5: Model of the DQC mechanism.
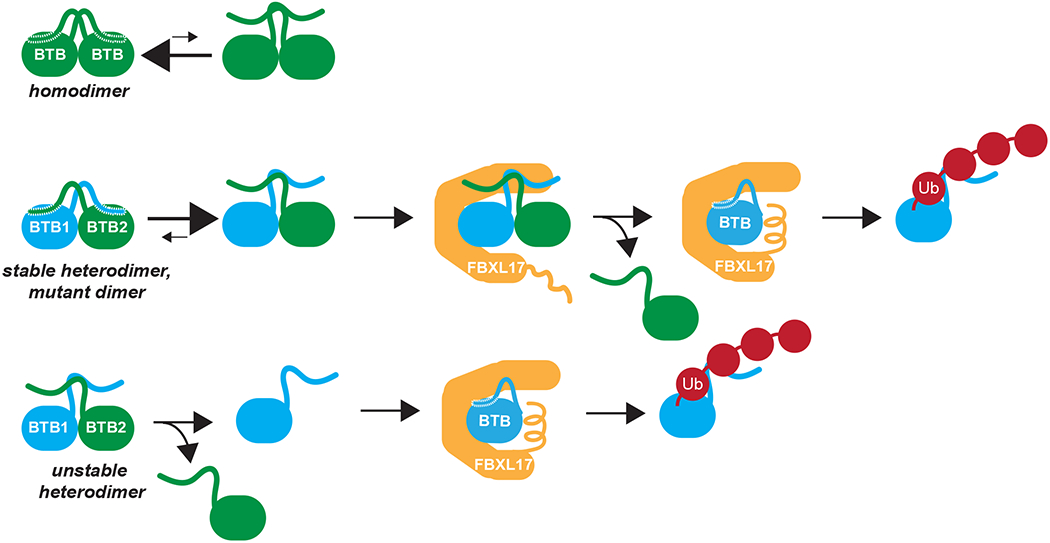
BTB homodimers have identical amino-terminal β-strand mostly in the domain swapped position. This prevents SCFFBXL17 from engaging and ubiquitylating the homodimer. BTB heterodimers or mutant BTB dimers have poorly compatible helices and β-strands. Their amino-terminal β-strand will be mostly displaced, which allows for capture of these aberrant dimers by SCFFBXL17. SCFFBXL17 could further destabilize these dimers or rely on spontaneous dimer dissociation to associate with a monomeric BTB subunit for ubiquitylation and degradation.
