Abstract
Background
This study aimed to assess the effects of sarcopenia and A Body Shape Index (ABSI) on cardiovascular disease (CVD) risk according to obesity phenotypes.
Methods
We used data from the National Health and Nutrition Examination Survey 1999 to 2012. A total of 25,270 adults were included and classified into the following groups: metabolically healthy normal weight (MHNW), metabolically healthy overweight/obese (MHO), metabolically unhealthy normal weight (MUNW), and metabolically unhealthy overweight/obese (MUO). Sarcopenia was defined as the appendicular skeletal mass index <7 kg/m2 in men and <5.5kg/m2 in women. A multivariate logistic regression analysis was performed to evaluate the odds ratio (OR) of sarcopenia and ABSI for CVD events according to the obesity phenotype.
Results
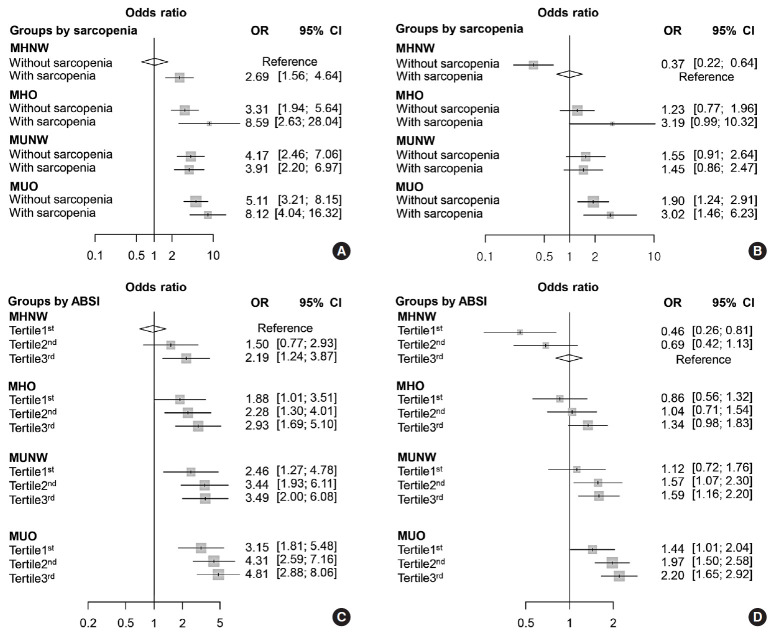
The MHNW participants with sarcopenia had higher risk for CVD than those without sarcopenia (OR, 2.69; 95% confidence interval [CI], 1.56 to 4.64). In the analysis with MHNW participants without sarcopenia as a reference, the participants with sarcopenia showed a higher OR for CVD than those without sarcopenia in both MHO (OR in participants without sarcopenia, 3.31; 95% CI, 1.94 to 5.64) (OR in participants with sarcopenia, 8.59; 95% CI, 2.63 to 28.04) and MUO participants (OR in participants without sarcopenia, 5.11; 95% CI, 3.21 to 8.15) (OR in participants with sarcopenia, 8.12; 95% CI, 4.04 to 16.32). Participants within the second and third tertiles of ABSI had higher ORs for CVDs than the counterpart of obesity phenotypes within the first tertile.
Conclusion
These results suggest that clinical approaches that consider muscle and body shape are required.
Keywords: Body size; Obesity, metabolically benign; Sarcopenia
INTRODUCTION
Obesity remains a leading cause of type 2 diabetes mellitus (DM), cardiovascular diseases (CVDs), and stroke and has substantially increased the public health burden [1,2,3,4]. To evaluate obesity accurately, direct fat mass measurement should be performed with computed tomography, magnetic resonance imaging, whole body dual-energy X-ray absorptiometry (DXA), or positron emission tomography-computed tomography [5]; however, direct measurements such as these are expensive and require modalities with limited availability in the clinical setting. Therefore, body mass index (BMI) has traditionally been used for the classification of obesity. However, one limitation of this method is that it cannot distinguish muscle from fat [6]. Recently, a number of studies found that low muscle mass; i.e., sarcopenia, was also associated with CVD [7]. Furthermore, epidemiologic studies suggested that a combination of sarcopenia and obesity has been associated with CVD and mortality [7].
Although obesity is usually associated with cardio-metabolic risk factors, not all obese individuals develop metabolic dysfunction. For example, some have a metabolically healthy overweight/obese (MHO) phenotype. While a recent meta-analysis of 19 studies showed that individuals with MHO were at a high risk of CVD, nine of studies did not produce statistically significant results [8] and the exact mechanism regarding this phenomenon remains unclear. Therefore, the inability of BMI to distinguish between fat and muscle may be one of the reasons results regarding the risk of CVD in individuals with MHO are heterogeneous. However, there have been no studies to assess the associations between muscle mass and CVD according to obesity phenotype.
Considering the limited availability of DXA in a clinical setting and the inability of BMI to estimate muscle mass, new obesity-related parameters that can distinguish between fat and muscle are needed. “A Body Shape Index” (ABSI) is a recently introduced parameter that reflects the body shape using waist circumference (WC), weight, and height [9]. Interestingly, ABSI was previously shown to have an inverse relationship with fat-free mass [10]; a modified ABSI also showed a positive association with the fat mass index as well as a negative association with the appendicular skeletal mass index (ASMI) [11]. Therefore, ABSI might supplement BMI by assessing muscle and fat mass.
In the present study, we assessed the effects of sarcopenia as defined by whole body DXA and body shape on CVD risk according to obesity phenotypes in a representative population in the United States.
METHODS
Study population
We used data from the National Health and Nutrition Examination Survey (NHANES) collected between 1999 and 2012. This survey covers a representative sample from the United States and has been performed biannually by the National Center for Health Statistics since 1999 using questionnaire-based personal interviews, physical examinations, and laboratory tests. Among a population of 71,916, those 20 years of age or younger as well as those who lacked questionnaire-based data on CVD events; weight, height, and blood pressure (BP) measurements; and/or laboratory data including fasting glucose, triglycerides, and high-density lipoprotein were excluded. Ultimately, 25,270 adults were included in this study (Supplementary Fig. 1). Whole body DXA data were available between 1999 and 2005; therefore, 11,317 participants were included in the subgroup analysis for body composition.
Measurements
WC was measured by placing a flexible tape around the uppermost lateral border of the ilium. BMI was defined as the weight (kg) divided by the square of the height (m). BP was measured three times after a minimum of 5 minutes of rest while the participant was in a sitting position, and the mean value of the three measurements was used in our analysis. Fasting blood glucose and lipid levels were measured using the enzymatic method. More detailed sample collection and test methods are described in the NHANES Laboratory Procedures Manual [12].
Definition of the obesity phenotypes and CVD
In the present study, overweight/obesity was defined as a BMI above 25 kg/m2 based on the World Health Organization criteria [13]. Owing to lack of a standard definition for metabolically healthy status, the revised National Cholesterol Education Program–Adult Treatment Panel III criteria (NCEP–ATP III criteria) for metabolic syndrome, which are the most commonly used criteria in previous studies [14,15,16,17], were also used. Metabolic abnormality was defined as having two or more metabolic risk factors according to the revised NCEP–ATP III criteria, including impaired fasting glucose (i.e., a fasting glucose level >100 mg/dL or a diagnosis of DM), high BP (hypertensive BP >130 mm Hg and/or diastolic BP >85 mm Hg, or a diagnosis of hypertension), triglyceride level ≥150 mg/dL, and a high-density lipoprotein cholesterol level <40 mg/dL in men and <50 mg/dL in women. Owing to the collinearity between BMI and WC, the central obesity criterion was not used. Based on these aforementioned criteria, the participants were classified into the following groups: metabolically healthy normal weight (MHNW), MHO, metabolically unhealthy normal weight (MUNW) and metabolically unhealthy overweight/obese (MUO). We identified the existence of a CVD event if 1 or more of the following structured questions were answered in the affirmative:
1. Has a doctor ever told you that you have congestive heart failure?
2. Has a doctor ever told you that you have coronary heart disease?
3. Has a doctor ever told you that you had a heart attack (or myocardial infarction)?
4. Has a doctor ever told you that you have angina pectoris?
5. Has a doctor ever told you that you have cerebrovascular disease?
Measurement of body composition and ABSI
Among the 25,270 study participants, 11,317 underwent a whole body DXA using a QDR 4500A fan beam X-ray bone densitometer (Hologic Inc., Marlborough, MA, USA). The total and regional body compositions as measured using the DXA scans were analyzed using the Hologic Discovery software version 12.1 (Hologic Inc.). The appendicular skeletal mass was defined as the sum of the total lean mass excluding bone mineral contents from the limbs, and the ASMI was defined as the appendicular skeletal mass divided by the square of the height (m). Sarcopenia was defined as an ASMI <7 kg/m2 in men and <5.5kg/m2 in women based on the revised European consensus on the definition and diagnosis of sarcopenia [18]. ABSI was calculated using the following equation: ABSI=WC/(BMI2/3×height1/2) [9].
Statistical analysis
The NHANES is a complex sample survey representing the United States population; the sample analysis was conducted using weighted values. Continuous variables are shown as mean values and 95% confidence intervals (CIs), while categorical variables are shown as the prevalence and percentage according to each obesity phenotype. Each variable was compared using 1-way analysis of variance and Pearson's chi-square test. We implemented Pearson's correlation coefficient to verify the correlation of ABSI with ASMI, BMI, and WC. A receiver operating characteristic (ROC) curve was used to analyze the correlation between ABSI and sarcopenia according to obesity phenotypes. A multivariate logistic regression analysis was performed to evaluate the odds ratio (OR) for CVD events according to the obesity phenotype. To determine the effects of muscle mass, groups were divided according to the presence of sarcopenia, and each group's OR for a CVD event was examined. Moreover, ABSI was divided into three categories, and the ORs for CVD events were calculated. Furthermore, changes in the ORs for CVD events in each obesity phenotype as a function of changes in ABSI were analyzed using the restricted cubic spline plots with 3 knots. The analysis was performed using R version 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org) and IBM SPSS Statistics version 24.0 (IBM Co., Armonk, NY, USA). A P<0.05 indicated statistical significance.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board of Hallym University Kangnam Sacred Heart Hospital (IRB No. HKS 2017-07-007). All the NHANES protocols were approved by the Research Ethics Review Board of the National Center for Health Statistics, US Centers for Disease Control and Prevention (NCHS IRB/ERB protocol numbers: 1999–2004, Protocol #98-12; 2005–2010, Protocol #2005-06; and 2011–2012, Protocol #2011-17). All participants volunteered and provided written informed consent prior to their enrollment. All participants' records were anonymized before being accessed by the authors, and all methods were performed in accordance with approved guidelines and regulations.
RESULTS
Baseline characteristics
The baseline characteristics of the participants according to their obesity phenotypes are summarized in Table 1. Of the 25,270 participants assessed in this study, 5,176 had MHNW, 6,688 were MHO, 2,671 were MUNW, and 10,735 were MUO. Compared to metabolically healthy participants, those metallically unhealthy participants were more likely to be men and of older age; they also had higher BP, poorer lipid and glucose profiles, and a higher prevalence of CVD. Metabolically unhealthy participants had higher ABSIs than metabolically healthy counterparts. DXA data were available for 11,317 of the 25,270 subjects; participants who were obese had significantly higher ASMIs than those who were not. Moreover, participants with normal weight showed a higher prevalence of sarcopenia than obese participants did.
Table 1.
Characteristics of the participants according to obesity phenotype
| Variable | Metabolically healthy |
Metabolically unhealthy |
P value | |||
|---|---|---|---|---|---|---|
| Normal weight (MHNW) (n=5,176) | Overweight/obesity (MHO) (n=6,688) | Normal weight (MUNW) (n=2,671) | Overweight/obesity (MUO) (n=10,735) | |||
| Age, yr | 38.2 (37.6–38.8) | 40.9 (40.4–41.5) | 54.7 (53.8–55.5) | 51.7 (51.3–52.2) | <0.001 | |
| Men, % | 39.6 (38.0–41.2) | 47.6 (46.3–49.0) | 48.1 (45.8–50.3) | 54.1 (53.0–55.2) | <0.001 | |
| Ethnicity/Race, % | <0.001 | |||||
| Mexican American | 6.2 (5.4–7.2) | 9.2 (7.7–10.9) | 4.6 (3.8–5.5) | 8.2 (6.7–9.8) | ||
| Other Hispanic | 4.3 (3.3–5.4) | 6.1 (4.8–7.7) | 4.4 (3.2–6.1) | 5.4 (4.3–6.9) | ||
| Non-Hispanic White | 73.9 (71.7–75.9) | 67.6 (64.7–70.3) | 73.4 (70.3–76.3) | 71.9 (69.1–74.6) | ||
| Non-Hispanic Black | 8.4 (7.4–9.6) | 13.2 (11.6–15) | 7.5 (6.4–8.9) | 10.2 (8.9–11.7) | ||
| Other race | 7.2 (6.2–8.3) | 4 (3.3–4.8) | 10 (8.3–12.1) | 4.3 (3.6–5.1) | ||
| CVD eventsa, % | 2.3 (1.8–2.9) | 4 (3.5–4.6) | 12.3 (10.9–13.9) | 12.5 (11.7–13.4) | ||
| Smoking, % | 45.4 (43.3–47.6) | 43.8 (42.1–45.7) | 55.5 (53–58.1) | 51.3 (49.9–52.7) | ||
| BMI, kg/m2 | 22 (22–22.1) | 30.2 (30–30.3) | 22.6 (22.5–22.7) | 32 (31.8–32.1) | <0.001 | |
| Waist circumference, cm | 80.7 (80.4–81) | 100.3 (99.9–100.8) | 85.5 (85.1–85.9) | 107.3 (106.8–107.7) | <0.001 | |
| ABSI | 0.0791 (0.0789–0.0793) | 0.0798 (0.0796–0.0800) | 0.0826 (0.0823–0.0828) | 0.0823 (0.0821–0.0824) | <0.001 | |
| ASMIa, kg/m2 | 6.55 (6.49 – 6.62) | 8.16 (8.10 – 8.22) | 6.45 (6.37 – 6.54) | 8.30 (8.24 – 8.36) | <0.001 | |
| Sarcopenia, % | 27.0 (24.4–29.8) | 1.4 (1.0–1.9) | 38.8 (35.9–41.8) | 2.1 (1.7–2.7) | <0.001 | |
| Systolic BP, mm Hg | 112 (111.5–112.5) | 115.6 (115.2–116) | 131 (129.8–132.2) | 129.1 (128.5–129.6) | <0.001 | |
| Diastolic BP, mm Hg | 67.9 (67.5–68.4) | 69.7 (69.3–70.1) | 72.5 (71.8–73.2) | 73.9 (73.5–74.4) | <0.001 | |
| FBG level, mg/dL | 90.8 (90.4–91.2) | 93.5 (93–94.1) | 105.9 (104.4–107.5) | 114.1 (112.8–115.4) | <0.001 | |
| HbA1c, % | 5.2 (5.2–5.2) | 5.3 (5.3–5.3) | 5.6 (5.6–5.7) | 5.9 (5.8–5.9) | <0.001 | |
| Total cholesterol, mg/dL | 185.7 (184.3–187.2) | 193.4 (192.2–194.6) | 209.2 (206.9–211.4) | 208.5 (207.1–209.8) | <0.001 | |
| HDL-C, mg/dL | 61.4 (60.8–62) | 54.9 (54.4–55.3) | 56.4 (55.3–57.5) | 46.5 (46.1–46.9) | <0.001 | |
| TG, mg/dL | 89.5 (86.7–92.3) | 107.9 (105.4–110.5) | 141.8 (131.7–151.8) | 183.3 (177.6–189.1) | <0.001 | |
| Metabolic state, % | ||||||
| High BP | 5.2 (4.5–6) | 6.8 (6–7.7) | 59.8 (57.2–62.4) | 51.4 (49.9–52.8) | <0.001 | |
| Hyperglycemia | 4.1 (3.5–4.7) | 5.9 (5.2–6.6) | 44.4 (41.7–47.1) | 55.6 (54–57.2) | <0.001 | |
| Low HDL-C level | 9.3 (8.2–10.4) | 13.1 (12–14.2) | 34 (31.4–36.8) | 52.5 (51.2–53.9) | <0.001 | |
| High TG level | 37.5 (35.9–39.2) | 48.3 (46.5–50) | 91.9 (90.4–93.2) | 91.8 (91–92.5) | <0.001 | |
Values are presented as mean (95% confidence interval).
MHNW, metabolically healthy normal weight; MHO, metabolically healthy overweight/obese; MUNW, metabolically unhealthy normal weight; MUO, metabolically unhealthy overweight/obese; CVD, cardiovascular disease; BMI, body mass index; ABSI, A Body Shape Index; ASMI, appendicular skeletal mass index; BP, blood pressure; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride.
Participants who had either angina pectoris, coronary heart disease, myocardial infarction, congestive heart failure, or cerebrovascular disease.
Association between obesity phenotypes and the risk of CVD events
A total of 2,637 CVD events were reported. In the multivariate logistic regression model with adjustment for age, sex, ethnicity and smoking status, participants with MHO were at a moderate risk of developing CVD when compared with MHNW participants (OR, 1.543; 95% CI, 1.158 to 2.056), whereas participants with metabolic abnormalities were at high risk regardless of obesity status (OR in MUNW, 2.026; 95% CI, 1.539 to 2.667) (OR in MUO, 2.725; 95% CI, 2.186 to 3.398) (Table 2).
Table 2.
Weighted odds ratios for cardiovascular disease events according to obesity phenotypes
| Variable | OR (95% CI) | P value |
|---|---|---|
| MHNW | 1 (reference) | |
| MHO | 1.543 (1.158–2.056) | 0.003 |
| MUNW | 2.026 (1.539–2.667) | <0.001 |
| MUO | 2.725 (2.186–3.398) | <0.001 |
Adjusted for age, sex, ethnicity, and smoking.
OR, odds ratio; CI, confidence interval; MHNW, metabolically healthy normal weight; MHO, metabolically healthy overweight/obese; MUNW, metabolically unhealthy normal weight; MUO, metabolically unhealthy overweight/obese.
Correlation of ABSI with ASMI and other obesity-related parameters
ABSI showed a moderate negative correlation with ASMI as well as a strong positive correlation with WC, regardless of obesity phenotype (Table 3). In contrast to ABSI, WC and BMI both showed a strong positive correlation with ASMI (overall correlation coefficient for WC, 0.631, P<0.001; overall correlation coefficient for BMI, 0.655, P<0.001). The area under the ROC curve (AUC) for ABSI showed significant accuracy for identifying sarcopenia (Table 4).
Table 3.
Correlation between ABSI and ASMI, BMI, and waist circumference
| Group | Correlation coefficients |
||
|---|---|---|---|
| ASMI | BMI | Waist circumference | |
| MHNW | |||
| Men | –0.586b | 0.039 | 0.695b |
| Women | –0.332b | –0.039a | 0.660b |
| MHO | |||
| Men | –0.400b | 0.082b | 0.552b |
| Women | –0.285b | –0.132b | 0.403b |
| MUNW | |||
| Men | –0.596b | 0.036 | 0.711b |
| Women | –0.283b | –0.043 | 0.694b |
| MUO | |||
| Men | –0.433b | –0.024 | 0.399b |
| Women | –0.351b | –0.249b | 0.251b |
ABSI, A Body Shape Index; ASMI, appendicular skeletal mass index; BMI, body mass index; MHNW, metabolically healthy normal weight; MHO, metabolically healthy overweight/obese; MUNW, metabolically unhealthy normal weight; MUO, metabolically unhealthy overweight/obese.
P<0.05,
P<0.001.
Table 4.
Area under the receiver operating characteristics curve of ABSI for sarcopenia
| Men |
Women |
|||
|---|---|---|---|---|
| AUC (95% CI) | P value | AUC (95% CI) | P value | |
| MHNW | 0.755 (0.722–0.787) | 0.017 | 0.677 (0.646–0.708) | 0.016 |
| MHO | 0.884 (0.802–0.966) | 0.042 | 0.722 (0.644–0.800) | 0.040 |
| MUNW | 0.776 (0.737–0.815) | 0.020 | 0.635 (0.589–0.681) | 0.023 |
| MUO | 0.854 (0.818–0.889) | 0.018 | 0.672 (0.613–0.732) | 0.030 |
ABSI, A Body Shape Index; AUC, area under curve; CI, confidence interval; MHNW, metabolically healthy normal weight; MHO, metabolically healthy overweight/obese; MUNW, metabolically unhealthy normal weight; MUO, metabolically unhealthy overweight/obese.
Effect of sarcopenia and ABSI on the risk of CVDs according to obesity phenotypes
The multivariate logistic regression analysis showed that the participants with sarcopenia had higher risk for CVD than those without sarcopenia in the group with MHNW (OR, 2.69; 95% CI, 1.56 to 4.64). The obese participants with sarcopenia showed a significantly higher ORs of CVD than those without sarcopenia in both the metabolically healthy and unhealthy groups (Fig. 1). Fig. 1A shows each OR according to obesity phenotype and sarcopenia status compared to MHNW participants without sarcopenia as a reference. The information in Fig. 1B is based on MHNW participants with sarcopenia as a reference. MHO participants had a significantly higher risk for CVD than did MHNW participants without sarcopenia (OR in MHO without sarcopenia, 3.31; 95% CI, 1.94 to 5.64) (OR in MHO with sarcopenia, 8.59; 95% CI, 2.63 to 28.04) (Fig. 1A). However, MHO participants did not have a significantly higher risk for CVD compared to the MHNW participants with sarcopenia (OR in MHO without sarcopenia, 1.23; 95% CI, 0.77 to 1.96) (OR in MHO with sarcopenia, 3.19; 95% CI, 0.99 to 10.23) (Fig. 1B).
Fig. 1. Weighted odds ratio (OR) (95% confidence interval [CI]) for cardiovascular events in terms of (A, B) obesity phenotype and sarcopenia, or (C, D) tertiles of A Body Shape Index (ABSI). (A) Compared to metabolically healthy normal weight (MHNW) without sarcopenia, (B) compared to MHNW with sarcopenia, (C) compared to MHNW with the first ABSI tertile, and (D) compared to MHNW with the third ABSI tertile. Values were adjusted for age, sex, ethnicity, and smoking status. MHO, metabolically healthy overweight/obese; MUNW, metabolically healthy normal weight; MUO, metabolically unhealthy overweight/obese.
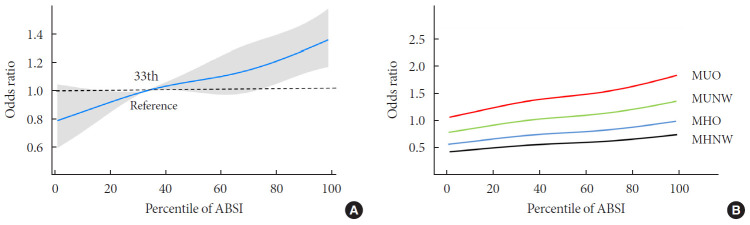
The multivariate logistic regression analysis showed that MHO participants who were within the second and third tertiles of ABSI had significantly higher ORs for CVDs, while those who were within the first tertile of ABSI had only a slightly higher risk for CVD, compared to the MHNW participants who were within the first ABSI tertile (Fig. 1C). However, MHO participants did not have a significantly higher risk of CVD compared to the MHNW participants within the third ABSI tertile (OR in MHO participants within the first tertile, 0.86; 95% CI, 0.56 to 1.32) (OR in MHO participants within the second tertile, 1.04; 95% CI, 0.71 to 1.54) (OR in MHO participants within the third tertile, 1.34; 95% CI, 0.98 to 1.84) (Fig. 1D). On restricted cubic spline regression analysis, ORs for CVD increased by ABSI in each obesity phenotype (Fig. 2).
Fig. 2. Relationship between continuous A Body Shape Index (ABSI) and the odds ratios for cardiovascular events according to obesity phenotypes. (A) Total population, and (B) obesity phenotype subgroups. Values were adjusted for age, sex, ethnicity, and smoking status. MUO, metabolically unhealthy overweight/obese; MUNW, metabolically unhealthy normal weight; MHO, metabolically healthy overweight/obese; MHNW, metabolically healthy normal weight.
DISCUSSION
The present study showed that sarcopenia was associated with a high risk of CVD in MHNW participants. We also observed that sarcopenia increased the risk of CVD even in obese participants regardless of metabolic abnormalities. Our large-scale data also revealed that ABSI is associated with sarcopenia and CVD risk according to obesity phenotypes.
Notably, our results in MHNW participants with sarcopenia illustrated the need for taking into account muscle mass measurements when defining normal weight with BMI. Numerous epidemiological studies have found that sarcopenia is associated with insulin resistance [19], type 2 DM [20], increased risk of CVD-related mortality, and all-cause mortality [21,22,23]. Our results were therefore consistent with those of previous studies. In addition, many studies showed that a combination of sarcopenia and obesity was more likely associated with metabolic disorders and morbidity [24,25,26,27,28]. A recent meta-analysis found that sarcopenic obesity increased the risk of all-cause mortality by 21% [29]. Our results found that obese patients with sarcopenia had a higher risk of CVD than those without sarcopenia in both the metabolically healthy and unhealthy group.
In the case of MHO phenotypes, several large-scale population-based studies have found an association between MHO and CVD risk [8], whereas several observational studies showed that MHO did not increase the risk of coronary atherosclerosis [30] or heart failure [31]. Our data suggested that if one study had an MHNW group with a high prevalence of sarcopenia as a control, their MHO phenotypes were not likely associated with an increased risk of CVD. Our data also revealed that if a study had an MHNW group with a low prevalence of sarcopenia as a control, their MHO phenotypes showed a remarkably higher risk for CVD. Therefore, the heterogeneity of information regarding prognoses in the various obesity studies could, at least in part, be due to overlooking the muscle mass measurement in their cohorts.
Our result also revealed that ABSI was positively correlated with WC but inversely correlated with the ASMI and had moderate accuracy for identifying sarcopenia. This result is consistent with recent studies which found an association between ABSI and sarcopenic obesity [10,11,32,33]. Considering limited availability of DXA in a clinical setting and the inability of BMI for estimating muscle mass, ABSI (which is easily obtained from the weight, height, and WC) is a useful alternative for identifying individuals who are at risk of sarcopenic obesity.
Several studies have examined the ABSI value as a predictor of CVD and mortality [9,34,35,36]. Moreover, a recent meta-analysis of 38 studies found that ABSI outperformed BMI and WC as a reliable predictor of all-cause mortality [37]. However, there is insufficient data on the association between ABSI and CVD according to obesity phenotypes. Only one study showed that a high level of modified ABSI increased the risk of CVD across obesity phenotypes using a Korean representative sample [38]. These data are consistent with our study using the United States representative sample. Considering the association between ABSI and sarcopenic obesity, the heterogeneity of CVD risk by ABSI in each obesity phenotype might reflect the influence of muscle loss on CVD.
There were limitations to our study. First, the fact that it was cross-sectional indicates that additional prospective studies are necessary to clarify the relationship between each obesity phenotype and CVD events. Second, fatal CVD events may have been missed given that we did not assess mortality data. However, our study is the first study to investigate the association between sarcopenia and CVD events according to obesity phenotypes in the recent national representative sample. Our findings might provide one explanation for the heterogeneous results produced by previous studies with respect to the risk of developing CVD in obese individuals [8,39,40].
In conclusion, obese participants with sarcopenia had a higher risk of CVD than those without sarcopenia, regardless of metabolic abnormalities. We also showed that sarcopenia increased the risk of CVD even in healthy participants and these results persisted in ABSI. These results suggest that clinical approaches that consider muscle mass are required. However, since whole body DXA cannot be applicable to measure muscle mass in most clinical setting, ABSI is a useful alternative for identifying obese individuals who are at risk of sarcopenia and CVD. However, additional prospective studies must be conducted to determine the effect of muscle mass and ABSI on CVD according to the obesity phenotype.
ACKNOWLEDGMENTS
None
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: S.M., J.G.K.
Acquisition, analysis, or interpretation of data: W.C., S.M., O.H.R.
Drafting the work or revising: H.W.C., W.C., S.M., M.K.K., J.G.K.
Final approval of the manuscript: H.W.C., W.C., S.M., O.H.R., M.K.K., J.G.K.
FUNDING
This work was supported by Research Resettlement Fund for the new faculty of Seoul National University and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (grant No. 2017R1D1A1B03029575).
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2019.0223.
Flowchart showing the selection process for the study's population. NHANES, National Health and Nutrition Examination Survey; CVD, cardiovascular disease.
References
- 1.Solomon CG, Manson JE. Obesity and mortality: a review of the epidemiologic data. Am J Clin Nutr. 1997;66(4 Suppl):1044S–1050S. doi: 10.1093/ajcn/66.4.1044S. [DOI] [PubMed] [Google Scholar]
- 2.Krauss RM, Winston M, Fletcher BJ, Grundy SM. Obesity: impact on cardiovascular disease. Circulation. 1998;98:1472–1476. [PubMed] [Google Scholar]
- 3.Moon S, Oh CM, Choi MK, Park YK, Chun S, Choi M, et al. The influence of physical activity on risk of cardiovascular disease in people who are obese but metabolically healthy. PLoS One. 2017;12:e0185127. doi: 10.1371/journal.pone.0185127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallstrom P, Bjartell A, Gullberg B, Olsson H, Wirfalt E. A prospective Swedish study on body size, body composition, diabetes, and prostate cancer risk. Br J Cancer. 2009;100:1799–1805. doi: 10.1038/sj.bjc.6605077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kushner RF. Clinical assessment and management of adult obesity. Circulation. 2012;126:2870–2877. doi: 10.1161/CIRCULATIONAHA.111.075424. [DOI] [PubMed] [Google Scholar]
- 6.Rey-Lopez JP, de Rezende LF, Pastor-Valero M, Tess BH. The prevalence of metabolically healthy obesity: a systematic review and critical evaluation of the definitions used. Obes Rev. 2014;15:781–790. doi: 10.1111/obr.12198. [DOI] [PubMed] [Google Scholar]
- 7.Kim TN, Choi KM. The implications of sarcopenia and sarcopenic obesity on cardiometabolic disease. J Cell Biochem. 2015;116:1171–1178. doi: 10.1002/jcb.25077. [DOI] [PubMed] [Google Scholar]
- 8.Mirzababaei A, Djafarian K, Mozafari H, Shab-Bidar S. The long-term prognosis of heart diseases for different metabolic phenotypes: a systematic review and meta-analysis of prospective cohort studies. Endocrine. 2019;63:439–462. doi: 10.1007/s12020-019-01840-0. [DOI] [PubMed] [Google Scholar]
- 9.Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS One. 2012;7:e39504. doi: 10.1371/journal.pone.0039504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biolo G, Di Girolamo FG, Breglia A, Chiuc M, Baglio V, Vinci P, Toigo G, Lucchin L, Jurdana M, Praznikar ZJ, et al. Inverse relationship between “a body shape index” (ABSI) and fat-free mass in women and men: insights into mechanisms of sarcopenic obesity. Clin Nutr. 2015;34:323–327. doi: 10.1016/j.clnu.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Chung W, Park JH, Chung HS, Yu JM, Kim DS, Moon S. Utility of the Z-score of log-transformed A Body Shape Index (LBSIZ) in the assessment for sarcopenic obesity and cardiovascular disease risk in the United States. Sci Rep. 2019;9:9292. doi: 10.1038/s41598-019-45717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. The National Health and Nutrition Examination Survey (NHANES) MEC laboratory procedures manual. [cited 2020 Mar 10]. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/manuals/2016_mec_laboratory_procedures_manual.pdf.
- 13.World Health Organization. Fact sheet on obesity and overweight. [cited 2020 Mar 10]. Available from: http://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight.
- 14.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 15.Jung CH, Lee MJ, Kang YM, Jang JE, Leem J, Hwang JY, et al. The risk of incident type 2 diabetes in a Korean metabolically healthy obese population: the role of systemic inflammation. J Clin Endocrinol Metab. 2015;100:934–941. doi: 10.1210/jc.2014-3885. [DOI] [PubMed] [Google Scholar]
- 16.Heianza Y, Arase Y, Tsuji H, Fujihara K, Saito K, Hsieh SD, et al. Metabolically healthy obesity, presence or absence of fatty liver, and risk of type 2 diabetes in Japanese individuals: Toranomon Hospital Health Management Center Study 20 (TOPICS 20) J Clin Endocrinol Metab. 2014;99:2952–2960. doi: 10.1210/jc.2013-4427. [DOI] [PubMed] [Google Scholar]
- 17.Hinnouho GM, Czernichow S, Dugravot A, Nabi H, Brunner EJ, Kivimaki M, et al. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: the Whitehall II cohort study. Eur Heart J. 2015;36:551–559. doi: 10.1093/eurheartj/ehu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One. 2010;5:e10805. doi: 10.1371/journal.pone.0010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim TN, Park MS, Yang SJ, Yoo HJ, Kang HJ, Song W, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS) Diabetes Care. 2010;33:1497–1499. doi: 10.2337/dc09-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han SS, Kim KW, Kim KI, Na KY, Chae DW, Kim S, et al. Lean mass index: a better predictor of mortality than body mass index in elderly Asians. J Am Geriatr Soc. 2010;58:312–317. doi: 10.1111/j.1532-5415.2009.02672.x. [DOI] [PubMed] [Google Scholar]
- 22.Wannamethee SG, Shaper AG, Lennon L, Whincup PH. Decreased muscle mass and increased central adiposity are independently related to mortality in older men. Am J Clin Nutr. 2007;86:1339–1346. doi: 10.1093/ajcn/86.5.1339. [DOI] [PubMed] [Google Scholar]
- 23.Atkins JL, Whincup PH, Morris RW, Lennon LT, Papacosta O, Wannamethee SG. Sarcopenic obesity and risk of cardiovascular disease and mortality: a population-based cohort study of older men. J Am Geriatr Soc. 2014;62:253–260. doi: 10.1111/jgs.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. 2008;18:388–395. doi: 10.1016/j.numecd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Choi KM. Sarcopenia and sarcopenic obesity. Endocrinol Metab (Seoul) 2013;28:86–89. doi: 10.3803/EnM.2013.28.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, Park YJ, et al. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA) Diabetes Care. 2010;33:1652–1654. doi: 10.2337/dc10-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu CW, Yang KC, Chang HH, Lee LT, Chen CY, Huang KC. Sarcopenic obesity is closely associated with metabolic syndrome. Obes Res Clin Pract. 2013;7:e301–e307. doi: 10.1016/j.orcp.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Choi KM. Sarcopenia and sarcopenic obesity. Korean J Intern Med. 2016;31:1054–1060. doi: 10.3904/kjim.2016.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Xie X, Dou Q, Liu C, Zhang W, Yang Y, et al. Association of sarcopenic obesity with the risk of all-cause mortality among adults over a broad range of different settings: a updated meta-analysis. BMC Geriatr. 2019;19:183. doi: 10.1186/s12877-019-1195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morkedal B, Vatten LJ, Romundstad PR, Laugsand LE, Janszky I. Risk of myocardial infarction and heart failure among metabolically healthy but obese individuals: HUNT (Nord-Trondelag Health Study), Norway. J Am Coll Cardiol. 2014;63:1071–1078. doi: 10.1016/j.jacc.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 31.Voulgari C, Tentolouris N, Dilaveris P, Tousoulis D, Katsilambros N, Stefanadis C. Increased heart failure risk in normal-weight people with metabolic syndrome compared with metabolically healthy obese individuals. J Am Coll Cardiol. 2011;58:1343–1350. doi: 10.1016/j.jacc.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 32.Gomez-Peralta F, Abreu C, Cruz-Bravo M, Alcarria E, Gutierrez-Buey G, Krakauer NY, et al. Relationship between “a body shape index (ABSI)” and body composition in obese patients with type 2 diabetes. Diabetol Metab Syndr. 2018;10:21. doi: 10.1186/s13098-018-0323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhana K, Koolhaas CM, Schoufour JD, Rivadeneira F, Hofman A, Kavousi M, et al. Association of anthropometric measures with fat and fat-free mass in the elderly: the Rotterdam study. Maturitas. 2016;88:96–100. doi: 10.1016/j.maturitas.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 34.Lee DY, Lee MY, Sung KC. Prediction of mortality with a body shape index in young Asians: comparison with body mass index and waist circumference. Obesity (Silver Spring) 2018;26:1096–1103. doi: 10.1002/oby.22193. [DOI] [PubMed] [Google Scholar]
- 35.Dhana K, Kavousi M, Ikram MA, Tiemeier HW, Hofman A, Franco OH. Body shape index in comparison with other anthropometric measures in prediction of total and cause-specific mortality. J Epidemiol Community Health. 2016;70:90–96. doi: 10.1136/jech-2014-205257. [DOI] [PubMed] [Google Scholar]
- 36.Bozorgmanesh M, Sardarinia M, Hajsheikholeslami F, Azizi F, Hadaegh F. CVD-predictive performances of “a body shape index” versus simple anthropometric measures: Tehran lipid and glucose study. Eur J Nutr. 2016;55:147–157. doi: 10.1007/s00394-015-0833-1. [DOI] [PubMed] [Google Scholar]
- 37.Ji M, Zhang S, An R. Effectiveness of A Body Shape Index (ABSI) in predicting chronic diseases and mortality: a systematic review and meta-analysis. Obes Rev. 2018;19:737–759. doi: 10.1111/obr.12666. [DOI] [PubMed] [Google Scholar]
- 38.Chung W, Park JH, Ryu OH, Yu JM, Yoo HJ, Moon S. Association of Z-score of the log-transformed a body shape index with cardiovascular disease in people who are obese but metabolically healthy: the Korea National Health and Nutrition Examination Survey 2007–2010. J Obes Metab Syndr. 2018;27:158–165. doi: 10.7570/jomes.2018.27.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arnlov J, Ingelsson E, Sundstrom J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation. 2010;121:230–236. doi: 10.1161/CIRCULATIONAHA.109.887521. [DOI] [PubMed] [Google Scholar]
- 40.Hinnouho GM, Czernichow S, Dugravot A, Batty GD, Kivimaki M, Singh-Manoux A. Metabolically healthy obesity and risk of mortality: does the definition of metabolic health matter? Diabetes Care. 2013;36:2294–2300. doi: 10.2337/dc12-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flowchart showing the selection process for the study's population. NHANES, National Health and Nutrition Examination Survey; CVD, cardiovascular disease.