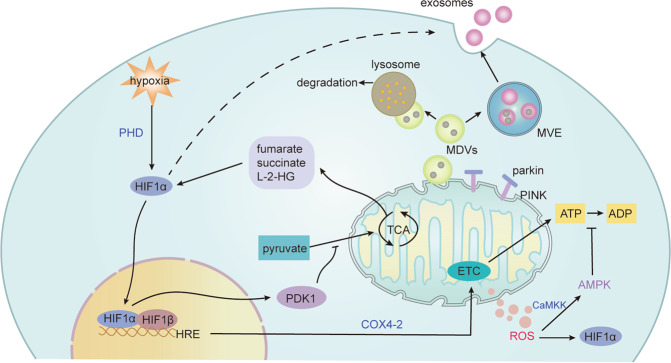
Fig. 2. Mitochondrial changes under hypoxia.
The tricarboxylic acid (TCA) cycle is inhibited under hypoxia, and HIF-1α prevents pyruvate from entering the TCA cycle by inducing pyruvate dehydrogenase kinase, isozyme 1 (PDK1). Hypoxia also regulates the activity of the electron transport chain (ETC). The switch of cytochrome C oxidase4 (COX4) subunits by HIF-1 activation for a more efficient transfer of electrons to O2 to meet energy demands in mammals under acute hypoxia, but sustained hypoxia can decrease ETC activity. The decreased activity of ETC limit the production of mROS, which mediates signal transduction and oxidative damage. The increase of mROS activates CaMKK, which activates AMPK to limit ATP consumption. Also, TCA cycle metabolites fumarate, succinate, L-2-hydroxyglutarate (L-2-HG), as well as mROS can cause the accumulation of HIFα. In addition, mitochondrial fission and fusion and mitophagy also play an important role in adaptation to hypoxia. Also, EVs and mitochondrial-derived vesicles (MDVs) can be effective mechanisms to maintain mitochondrial quality control. Damaged mitochondria and compositions can be removed by EVs. Also, mitochondrial stress induces the formation of MDVs partly depending on parkin and PINK1, which can fuse with multivesicular endosomes (MVEs) or lysosomes, and the contents can be secreted to the extracellular space via exosomes or degraded, respectively. Ca2+/calmodulin-dependent protein kinase kinase (CaMKK).