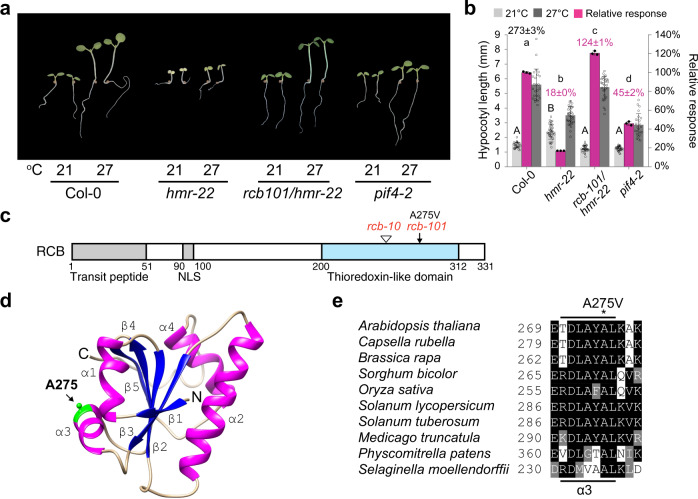
Fig. 1. Identification of rcb-101 as a suppressor of hmr-22 in thermomorphogenesis.
a Representative images of 4-d-old Col-0, hmr-22, rcb-101/hmr-22, and pif4-2 seedlings grown in 50 μmol m−2 s−1 R light at either 21 or 27 °C. b Hypocotyl length measurements of the seedlings in (a). The light- and dark-gray bars represent hypocotyl length measurements at 21 °C and 27 °C, respectively. The percent increase in hypocotyl length (mean ± s.d., n = 3 biological replicates) of Col-0 at 27 °C is shown in black above its columns. The magenta bars show the relative response, which is defined as the relative hypocotyl response to 27 °C of a mutant compared with that of Col-0 (set at 100%). Error bars for the hypocotyl measurements represent the s.d. (n > 30 seedlings); error bars for the relative responses represent the s.d. of three biological replicates. The centers of the error bars represent the mean values. Purple numbers show the mean ± s.d. values of relative responses and different lowercase letters denote statistically significant differences in relative responses (ANOVA, Tukey’s HSD, p < 0.01, n = 3 biological replicates). Different uppercase letters denote statistically significant differences in hypocotyl length at 21 °C (ANOVA, Tukey’s HSD, p < 0.01, n > 24 seedlings). c Schematic of the domain structure of RCB and the mutations in rcb-101 and rcb-10. NLS, nuclear localization signal. d Simulated structure of RCB’s thioredoxin-like domain highlighting Ala-275 in α336. e Amino acid sequence alignment of selected RCB orthologs showing that Ala-275 is highly conserved in land plants. The underlying source data of the hypocotyl measurements in (b) are provided in the Source Data file.