Figure 1.
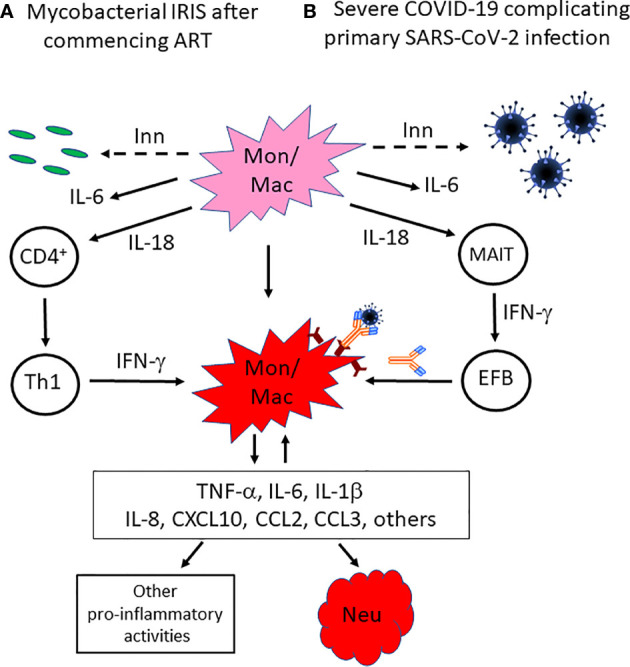
Proposed mechanisms by which innate immune responses not only induce inflammation but also skew emerging adaptive immune responses towards a response that amplifies myeloid cell activation in mycobacterial IRIS or COVID-19. In the absence of effective adaptive immune responses against (A) mycobacteria in HIV patients with severe CD4+ T cell deficiency and a mycobacterial co-infection, or (B) acute infection with the novel pathogen SARS-CoV-2, innate immune responses are generated that result in activation of monocytes/macrophages, including NLRP3-inflammasome activation. While this does not control the mycobacterial or SARS-CoV-2 infection, it generates a pro-inflammatory cytokine environment that includes IL-18, which skews CD4+ T cell recovery towards a Th1 response after ART is commenced in HIV patients, or induces MAIT cell activation that promotes B cell differentiation through an extra-follicular pathway and the production of a SARS-CoV-2 SP antibody response with pro-inflammatory characteristics, including decreased fucosylation of IgG1 Fc glycans and higher proportions of IgG3 and IgA2 antibodies, which enhances binding of SARS-CoV-2/antibody complexes to activatory FcγRs on macrophages, in COVID-19 patients. In either situation, dysregulated production and activity of multiple pro-inflammatory cytokines and chemokines occurs and has multiple effects, including further recruitment and activation of neutrophils and monocytes, as well as macrophage activation in a positive feedback loop. Inn, innate immune response; Mon/mac, monocytes/macrophages; Neu, neutrophils; MAIT, mucosa-associated invariant T cells; EFB, extra-follicular B cells.
