Abstract
Oxidative stress and oxidative damage are the common pathophysiological characteristics in pituitary adenomas (PAs), which have been confirmed with many omics studies in PA tissues and cell/animal experimental studies. Nuclear factor erythroid 2 p45-related factor 2 (Nrf2), the core of oxidative stress response, is an oxidative stress sensor. Nrf2 is synthesized and regulated by multiple factors, including Keap1, ERK1/2, ERK5, JNK1/2, p38 MAPK, PKC, PI3K/AKT, and ER stress, in the cytoplasm. Under the oxidative stress status, Nrf2 quickly translocates from cytoplasm into the nucleus and binds to antioxidant response element /electrophile responsive element to initiate the expressions of antioxidant genes, phases I and II metabolizing enzymes, phase III detoxifying genes, chaperone/stress response genes, and ubiquitination/proteasomal degradation proteins. Many Nrf2 or Keap1 inhibitors have been reported as potential anticancer agents for different cancers. However, Nrf2 inhibitors have not been studied as potential anticancer agents for PAs. We recommend the emphasis on in-depth studies of Nrf2 signaling and potential therapeutic agents targeting Nrf2 signaling pathways as new therapeutic strategies for PAs. Also, the use of Nrf2 inhibitors targeting Nrf2 signaling in combination with ERK inhibitors plus p38 activators or JNK activators targeting MAPK signaling pathways, or drugs targeting mitochondrial dysfunction pathway might produce better anti-tumor effects on PAs. This perspective article reviews the advances in oxidative stress and Nrf2-mediated oxidative stress response signaling pathways in pituitary tumorigenesis, and the potential of targeting Nrf2 signaling pathways as a new therapeutic strategy for PAs.
Keywords: pituitary adenoma, oxidative stress, Nrf2, signaling pathway, biomarker, therapeutic target and drug
Introduction
Pituitary adenoma (PA) is a common intracranial neoplasm that occurs in the central regulatory organ pituitary gland in the hypothalamic-pituitary-target organ axis system, which seriously affects human endocrine system and health. PAs account for 10–25% of all intracranial tumors, and are classified into benign (∼65%), invasive (∼35%), and malignant (carcinoma; only 0.1–0.2%) PAs according to the malignancy level (Stalla et al., 2019). PAs are divided into macroadenomas (≥10 mm) and microadenomas (<10 mm) according to tumor size (Lopes, 2017). They are also divided into clinically functional and nonfunctional PAs (FPAs and NFPAs) according to the level of hormone secretion (Zhan et al., 2016). FPAs are hormone-secreting PAs, which result in hyperpituitarism, including acromegaly derived from growth hormone (GH)-secreting PAs, hyperprolactinemia derived from prolactin (PRL)-secreting PAs, and Cushing’s syndrome derived from adrenocorticotropin (ACTH)-secreting PAs. NFPAs are non-hormone-secreting PAs (Qian et al., 2018). The main clinical symptoms of PAs include inappropriate hormone secretion syndrome, and compression of the neighboring tissues and structures such as headache, visual field defect, and increased intracranial pressure (Reimondo et al., 2019). PA is a multi-factor, multi-process, and multi-consequence complex disease, which is involved in a series of molecular alterations at the levels of genome, transcriptome, proteome, peptidome, metabolome, and radiome; and these molecules mutually associate and function in a molecular network system (Zhan and Desiderio, 2010b; Hu et al., 2013; Grech et al., 2015; Cheng and Zhan, 2017; Lu and Zhan, 2018). Thus, one must shift the research and practice strategy from a single-factor model to a multi-parameter systematic model for predictive, preventive, and personalized medicine in PAs (Hu et al., 2013; Grech et al., 2015; Cheng and Zhan, 2017). Multiomics is an effective approach to realize this multi-parameter systematic strategy model shift, which can establish signaling pathway systems for in-depth understanding of molecular mechanisms of PAs, identify molecular network-based biomarkers for prediction, diagnosis, and prognostic assessment of PAs, and discover signaling pathway network-based therapeutic targets for effective treatment of PAs (Grech et al., 2015; Cheng and Zhan, 2017; Lu and Zhan, 2018).
A series of omics analyses have been performed in PAs to reach our long-term goals that clarify molecular mechanisms and discover effective biomarkers and therapeutic targets for PAs (Zhan and Desiderio, 2010a; Long et al., 2019; Cheng et al., 2019; Wang Y. et al., 2019), including NFPA quantitative transcriptomics (differentially expressed genes, DEGs) (Moreno et al., 2005; Cheng et al., 2019), NFPA quantitative proteomics (differentially expressed proteins, DEPs) (Moreno et al., 2005), NFPA proteomic mapping (Zhan and Desiderio, 2003; Wang X. et al., 2015; Cheng et al., 2019), NFPA nitroproteomics (Zhan and Desiderio, 2006), invasive NFPA quantitative transcriptomics (Galland et al., 2010; Zhou et al., 2011; Wang Y. et al., 2019), invasive NFPA quantitative proteomics (Zhan et al., 2014b), control pituitary proteomic mapping (Beranova-Giorgianni et al., 2002; Giorgianni et al., 2003; Zhao et al., 2005), pituitary control nitroproteomics (Zhan and Desiderio, 2004; Zhan and Desiderio, 2007), control pituitary phosphoproteomics (Giorgianni et al., 2004; Beranova-Giorgianni et al., 2006), PRL-secreting adenoma proteomics and transcriptomics (Evans et al., 2008), and ACTH-secreting adenoma proteomics and metabolomics (Feng et al., 2018). Integrative analysis of these omics data has revealed some important signaling pathway network alterations in PA pathogenesis, including mitochondrial dysfunction, oxidative stress, cell cycle dysregulation, and mitogen-activated protein kinase (MAPK) signaling pathway alteration (Zhan and Desiderio, 2010a; Long et al., 2019). Mitochondrial dysfunction pathway network and mitochondrial dynamics (Li and Zhan, 2019), and MAPK signaling pathway-based drug therapeutic targets (Lu et al., 2019) have been discussed in detailed in PAs. It is well-known that mitochondria are the energy factories of the body, and mitochondrial metabolism is the source of reactive oxygen species (ROS). The imbalance between free radicals reactive oxygen/nitrogen species (ROS/RNS) and antioxidant system leads to oxidative stress, which plays an important role in diseases. Many studies focus on oxidative stress system as therapeutic strategy; for example, benfotiamine is an efficient antioxidant, which could prevent oxidative stress in the anterior tibialis muscle and heart of mice (Gonçalves et al., 2019). Another research shows that pancreatic oxidative damage in the diabetic state is caused by ROS, and scavenging the various ROS generated in the disease is one of effective ways to treat this disease (Afolabi et al., 2018). Studies have clearly demonstrated that mitochondrial dysfunction and oxidative stress pathway changes operate in PAs (Zhan and Desiderio, 2010a), and nuclear factor erythroid 2 p45-related factor 2 (Nrf2)-mediated oxidative stress response significantly impacts the pathogenesis of PAs and modulates the energy metabolism reprogramming for PAs (Sabatino et al., 2018). It is well-known that PAs can lead to abnormal hormone secretion, which might affect oxidative stress and Nrf2 signaling in PAs; for example, human growth hormone (hGH) can attenuate inflammation and oxidative stress attained by Cisplatin probably through inhibition of Nrf2/heme oxygenase 1 (HO-1) pathway (Mahran, 2020). More studies show that Nrf2 signaling and oxidative stress can be regulated by cortisol (Wu et al., 2019), thyroid hormone (Mishra et al., 2019), follicle-stimulating hormone (FSH) (Li et al., 2020), luteinizing hormone (LH) (Li et al., 2020), GH (Mahran, 2020), ACTH (Benlloch et al., 2016), and PRL (Ebokaiwe et al., 2020). These findings clearly demonstrate the importance of oxidative stress in PAs. This present review article will focus on oxidative stress response signing pathway network in PA pathogenesis.
Redox Homeostasis and Nrf2 as the Heart of Oxidative Stress Response
Oxidative stress is derived from the imbalance between the upload of free radicals ROS/RNS from in vivo and in vitro environmental approaches and the ability of endogenous antioxidants to detoxify these ROS/RNS (Prasad et al., 2016; Klaunig, 2018; Sajadimajd and Khazaei, 2018). It results in the injuries of multiple biomacromolecules such as DNAs, RNAs, proteins, and membrane lipids to significantly associate with a wide spectrum of diseases including cancers. Many studies demonstrate that the increased ROS/RNS productions promote carcinogenesis development (Kudryavtseva et al., 2016; Kruk and Aboul-Enein, 2017), and oxidative stress-medicated chronic inflammation is the risk factor of tumorigenesis (Reuter et al., 2010; Qian et al., 2019). The oxidative phosphorylation system in mitochondrial respiratory chain is the central machine that generates ROS products such as superoxide radical (O2 .-). One study shows that ROS levels and signs of oxidative damage are significantly increased in PAs (Sabatino et al., 2018). One of the most important RNS, nitric oxide (NO), is generated by inducible nitric synthase (iNOS) in many pathogenesis conditions, which can rapidly react with superoxide radical (O2 .-) to generate more toxic peroxynitrite anion (ONOO-) and highly reactive hydroxyl radical (OH.) to attack DNAs, RNAs, proteins, and membrane lipids. iNOS has been extensively found in rat and human pituitaries (Ceccatelli et al., 1993; Lloyd et al., 1995; Ueta et al., 1998; Kruse et al., 2002; Pawlikowshi et al., 2003) and has the elevated activities in PAs compared to those in controls (Vankelecom et al., 1997; Kruse et al., 2002). Another study shows that NO functions in the hypothalamic-pituitary-adrenocortical axis (Riedel, 2002) by promoting the release of follicle-stimulating hormone-releasing hormone (FSHRH) and luteinizing hormone-releasing hormone (LHRH) from hypothalamus (McCann et al., 2001; Pinilla et al., 2001; McCann et al., 2003), and regulating secretion of PRL (Duvilanski et al., 1995) and GH in pituitaries and PAs (Cuttica et al., 1997; Pinilla et al., 1999; Bocca et al., 2000). Peroxynitrite anion (ONOO−) is a key factor in vivo that causes protein tyrosine nitration and alters protein functions. Nine nitrotyrosine-containing proteins have been identified in NFPA tissues, and tyrosine nitration occurs in important structural and functional domains to change protein functions (Zhan and Desiderio, 2006).
With the generation of ROS/RNS, the in vivo antioxidant detoxification system is correspondingly initiated to adapt against the increased ROS/RNS (Valko et al., 2006; Obrador et al., 2019). The endogenous antioxidant detoxification system is a very complex system, including i) enzymatic antioxidants such as superoxide dismutases (CuZnSOD and MnSOD), glutathione peroxidase, and catalase; ii) non-enzymatic antioxidants such as vitamin E, vitamin C, carotenoid, flavonoid, selenium, thiol antioxidant (thioredoxin, lipoic acid, and glutathione), and others; and iii) multiple regulatory factors [Nrf2, NF-kB (nuclear factor kB), and AP-1 (activator protein-1), etc.] that interact with antioxidants (Valko et al., 2006; Obrador et al., 2019). CuZnSOD exists in most parts of cells, while MnSOD is only found in mitochondrial matrix; and both of them are able to effectively scavenge O2 .- and generate H2O2 (Li et al., 1995; Melov et al., 2001; Elchuri et al., 2005). H2O2 can be scavenged by GPX's (glutathione peroxidases) and peroxiredoxins (thioredox-independent peroxidases) (Chu et al., 2004; Kang et al., 2005). Studies have found that the levels of CuZnMOD and MnSOD are significantly lower in PAs compared to those of controls (Kurisaka et al., 2004; Yang et al., 2012; Ilhan et al., 2018). The abnormal activities of these antioxidant enzymes and non-enzymatic antioxidants are directly associated with carcinogenesis (Neumann et al., 2003; Chu et al., 2004; Harris et al., 2015). The transcription factor Nrf2 is pivotal to the antioxidant response, which is a sensor of oxidative stress in redox homeostasis, and is mainly located in the cytoplasm under basal conditions (Li and Kong, 2009; Furfaro et al., 2016a). When the upload of free radicals ROS/RNS is increased to cause oxidative stress, Nrf2 quickly translocates from cytoplasm into the nucleus to initiate the antioxidant response, protecting against oxidative/nitrative damages (Dhakshinamoorthy and Porter, 2004; Osburn et al., 2006; Mann et al., 2007; Pi et al., 2008). The Nrf2 signaling regulatory system contains at least four components, including Nrf2, Kelch-like ECH-associated protein 1 (Keap1), small musculoaponeurotic fibrosarcoma (Maf), and antioxidant response element (ARE) or electrophile responsive element (EpRE), which in combination are necessary for the antioxidant response (Kwak and Kensler, 2010; Furfaro et al., 2016; de la Vega et al., 2018). Nrf2 signaling pathways regulate multiple biological processes, including i) the expressions of antioxidant genes, ii) ubiquitin-proteasome system, iii) molecular chaperone/stress-response system, and iv) anti-inflammatory response (Kwak and Kensler, 2010; Furfaro et al., 2016). The accumulated evidence clearly demonstrates that Nrf2 signaling pathways are involved in 12 hallmarks of cancer, including sustained proliferative signaling, insensitivity to antigrowth signals, resistance to apoptosis, limitless replicative potential, sustained angiogenesis, tissue invasion and metastasis, metabolic reprogramming, avoiding immune destruction, tumor-promoting inflammation, genome instability, altered redox homeostasis, and proteotoxic stress (de la Vega et al., 2018). Thereby, any decreased capability of the antioxidant protective system in the redox homeostasis might cause more susceptibility to carcinogen toxicity, tumor inflammatory response, oxidative stress, and carcinogenesis (Yates and Kensler, 2007).
Multiuomics Reveals Oxidative Stress-Related Pathway Alterations in PAs
Our multiomics studies in PAs (Zhan and Desiderio, 2010a; Long et al., 2019) clearly demonstrate oxidative stress-related pathway changes in PAs. For example, i) Nrf2-mediated oxidative stress response pathway is significantly changed in NFPAs with evidence of upregulation of key molecules [upregulated DEPs: GST (glutathione S-transferase) or GSTM2 (glutathione S-transferase mu 2), and ERP29 (endoplasmic reticulum protein 29], and downregulation of key molecules [downregulated DEPs: HSP22 (heat shock protein 22), HSP27, and HSP90 or GRP94 (94 kD glucose-regulated protein)] in this pathway. ii) Mitochondrial dysfunction pathway is significantly changed in NFPAs with evidence of upregulation of key molecules [upregulated DEPs: NDUFS8 (NADH ubiquinone oxidoreductase core subunit S8), COX6B (cytochrome c oxidase subunit 6B), CAT (catalase), β-secret2, and ATP5B (ATP synthase, H+ transporting mitochondrial F1 complex, beta subunit)], and downregulation of key molecules [downregulated DEPs: GPX4 (glutathione peroxidase 4), and ATP5A1] in this pathway. Mitochondrial dysfunction can increase ROS production in cancer cells to mediate tumor-related signaling pathways and activate pro-oncogenic signaling (Li and Zhan, 2019). iii) Oxidative phosphorylation pathway is significantly changed in NFPAs with evidence of upregulation of key molecules (upregulated DEPs: NDUFS8, COX6B, and ATP5B) in this pathway. Mitochondrial oxidative phosphorylation system contains mitochondrial complexes I, II, III, IV, and V, which are the major sites that produce endogenous ROS such as OH. and O− 2; among these, complexes I, II, and III play a crucial role in the generation of mitochondrial ROS, because the electrons tend to be leaky at complexes I and III, which results in an incomplete reduction of oxygen and thus generates a free radical such as superoxide radical (Li and Zhan, 2019). iv) Glutathione redox reaction I pathway is significantly changed in NFPAs with evidence of downregulation of key molecule (downregulated DEP: GPX4) in this pathway. GPX’s (glutathione peroxidases) are important components in the antioxidant defense system: the downregulation of GPX’s can decrease the capability of the antioxidant defense system. v) The superoxide radical degradation pathway is significantly changed in NFPAs with evidence of upregulation of key molecule (upregulated DEP: CAT) in this pathway. vi) Aryl hydrocarbon receptor signaling is significantly changed in NFPAs with evidence of upregulation of key molecules [upregulated DEP: GST; upregulated DEGs: HSPCA (heat shock protein 90 alpha family class A member 1), HSPCB (heat shock protein 90 alpha family class B member 1), ESR1 (estrogen receptor 1), and Bax (BCL2 associated X, apoptosis regulator)], and downregulation of key molecules [downregulated DEPs: HSP27, HSP90 or GRP94, and TGM2 (transglutaminase 2); downregulated DEG: ESR2 (estrogen receptor 2)] in this pathway. vii) Glucocorticoid receptor signaling is significantly changed in NFPAs with evidence of upregulation of key molecule [upregulated DEG: PI3K (phosphatidylinositol 3 kinase)], and downregulation of key molecules [downregulated DEGs: HSP70, c-Fos, CCL2 (C-C motif chemokine ligand 2), BCL2, PRL, and POMC (proopiomelanocortin)] in this pathway. viii) Corticotropin-releasing hormone signaling is significantly changed in NFPAs with evidence of upregulation of key molecules [upregulated DEGs: CALM (calmodulin), and IP3R)], and downregulation of key molecules [downregulated DEGs: ACTH, Nur77 (NR4A1 nuclear receptor subfamily 4 group A member 1), and c-FOS)] in this pathway. ix) Melatonin signaling is significantly changed in NFPAs with evidence of nitration of key molecule (PKA) in this pathway. x) Methylglyoxal degradation III pathway is significantly changed in NFPAs with evidence of upregulation of key molecules [upregulated DEPs: aldose reductase or AKR1B1(aldo-keto reductase family 1 member B)] in this pathway. xi) AMPK signaling is significantly changed in NFPAs with evidence of upregulation of key molecules [upregulated DEGs: PP2C (putative protein phosphatase), and PFK (phosphofructokinase)], and downregulation of key molecules [downregulated DEGs: PI3K, PKA, and PDK1 (pyruvate dehydrogenase kinase 1)] in this pathway. Thereby, these signaling pathway changes clearly demonstrate that the disturbance in redox homeostasis, the imbalance between generation and detoxification of free radicals ROS/RNS, results in oxidative stress and damage in human PAs. Recently, these findings are also confirmed with experiments in cell models and animal models, which demonstrate that increased mitochondrial fusion results in bigger mitochondria, increased ROS levels, and oxidative damage in PAs, and that Nrf2 signaling pathway is activated in PAs as an antioxidant response (Sabatino et al., 2018). Thus, it suggests that Nrf2 is the master regulator of the cellular antioxidant response (de la Vega et al., 2018).
Nrf2-Mediated Oxidative Stress Response Signaling Pathways in PAs
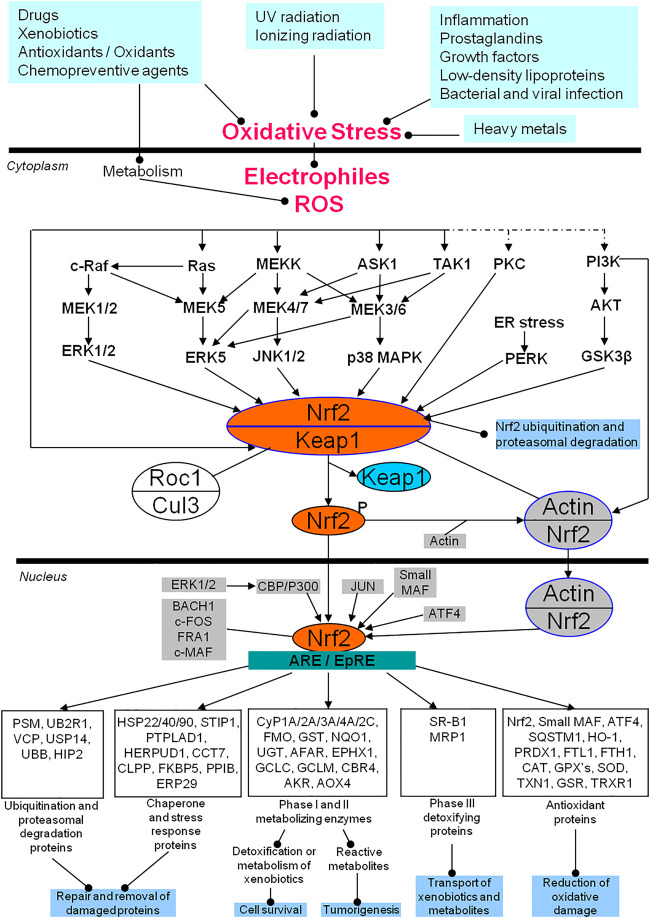
Nrf2 signaling pathway in response to oxidative stress is shown (Figure 1). Multiple in vivo and in vitro environmental factors, including inflammatory cytokines, prostaglandins, growth factors, low-density lipoproteins, bacterial and viral infection, heavy metals, ultraviolet (UV) radiation, ionizing radiation, drugs, xenobiotics, antioxidants, oxidants, and chemopreventive agents, cause the increased upload of free radicals ROS/RNS and electrophiles to result in oxidative stress (Hetland et al., 2020; Mehnati et al., 2020). The increased ROS or electrophiles will activate the Nrf2/Keap1 complex in the cytoplasm through ERK1/2, ERK5, JNK1/2, p38 MAPK, PKC, and PI3K-AKT signaling pathways, and these signaling pathways will communicate with each other (Roy Chowdhury et al., 2014; Tian et al., 2014; Wang K.-C. et al., 2019). The activated Nrf2 is phosphorylated and separated from Keap1 (Hambright et al., 2015; Sánchez-Martín et al., 2020). The separated and phosphorylated Nrf2 quickly translocates into the nucleus to interact with ARE or EpRE, which will initiate at least five types of gene expressions to exert the corresponding biological functions (Furfaro et al., 2016; Sánchez-Martín et al., 2020): i) reduction of the oxidative damage via antioxidant proteins such as NRF2, small MAF, ATF4, SQSTM1, HO-1, PRDX1, FTL, FTH1, CAT, GPX’s, SOD, TXN, GSR, and TRXR1 (Sun et al., 2019; Saad El-Din et al., 2020; Yu et al., 2020); ii) detoxification and metabolism of xenobiotics to regulate cell survival, or production of reactive metabolites to promote tumorigenesis via phase I and II metabolizing enzymes such as CYP1A/2A/3A/4A/2C, FMO, GST, NQD, UGT, AFAR, EPHX1, GCLC, GCLN, CBR4, AKR, and AOX4 (Zhao et al., 2015; Huang et al., 2018); iii) transportation of xenobiotics and metabolites via phase III detoxifying proteins such as SR-B1 and MRP1 (Sivils et al., 2013; Lubelska et al., 2016); iv) repairment and removal of the damaged proteins via chaperone and stress response proteins such as HSP22/40/90, STIP1, PTPLAD1, HERPUD1, CCT7, CLPP, FKBP5, PPIB, and ERP29 (Niture and Jaiswal, 2010; Sahin et al., 2012); and v) repairment and removal of the damaged proteins via ubiquitination and proteasomal degradation proteins such as PSM, UB2R1, VCP, USP14, UBB, and HIP2 (Liu et al., 2019; Song et al., 2019). This clearly demonstrates that while the Nrf2-mediated oxidative stress response signaling pathways are regulated by multiple factors, Nrf2 is the essential component. In the cytoplasm, Keap1, the main regulator of Nrf2, is a substrate adaptor protein for the Cul3-Keap1-E3 ligase complex that ubiquinates Nrf2, marking it for proteasomal degradation in the cytoplasm under basal conditions (Baird and Yamamoto, 2020; Dayalan Naidu and Dinkova-Kostova, 2020). To reduce its inhibitory effects on Nrf2, Keap1 can be ubiquitinated for degradation, leading to an increase in Nrf2 phosphorylation (activation) (Villeneuve et al., 2010). The phosphorylated Nrf2 can then interact with actin to form an Nrf2/actin complex that then translocates into the nucleus. After Nrf2 translocates into the nucleus, there are additional regulatory systems in place that include multiple factors such as ATF4, JUN, ERK1/2-CBP/P300, small MAF, BACH1, c-FOS, FRA1, and c-MAF, to influence the binding of Nrf2 and ARE/EpRE. The detailed regulatory mechanism system of Nrf2 has been extensively reviewed (Kwak and Kensler, 2010; Hybertson et al., 2011; Furfaro et al., 2016a; Lu et al., 2016; Menegon et al., 2016; Taguchi and Yamamoto, 2017; Bellezza et al., 2018; Chen and Maltagliati, 2018; de la Vega et al., 2018; Ryoo and Kwak, 2018; Sajadimajd and Khazaei, 2018; Cloer et al., 2019; Cuadrado et al., 2019; Qin et al., 2019) response signaling pathways have also been studied in pituitaries and PAs. One study shows that Nrf2, phosphorylated Nrf2 (p-Nrf2) protein, and mRNA expressions are increased in PAs, and the Nrf2 downstream effector HO-1 is also increased in PAs (Sabatino et al., 2018). This clearly demonstrates the activation of the Nrf2 signaling pathway, likely causing the extensive surviving capability of pituitary tumor cells. The Nrf2/PTEN-induced putative kinase protein 1 (PINK1)/Parkin pathway and mitophagy are activated in T-2 toxin-induced toxicities in rat pituitary GH3 cells (Deyu et al., 2018). Antioxidants N-acetylcysteine (NAC) and vitamin E can decrease the expressions of Nrf2 and HO-1 in rat pituitaries (Prevatto et al., 2017). Genetically induced Nrf2 overexpression in melanoma cells promotes tumor growth and increases antioxidant defense in malignant cells, which can be inhibited by anticancer agent pterostilbene (Pter, a natural dimethoxylated analog of resveratrol) through the downregulation of pituitary production of ACTH, plasma corticosterone, and the glucocorticoid receptor- and Nrf2-dependent antioxidant defense systems in growing melanomas (Benlloch et al., 2016). Irradiation can result in oxidative damage in C57/BL6 mice via activation of Nrf2 and HO-1 expressions, which can be blocked by antioxidant agent pituitary adenylate cyclase-activating polypeptide 38 (PACAP38) through inhibiting Nrf2 expression (Li et al., 2019). Chronic restraint stress (CRS) and acute restraint stress (ARS) can upregulate the mRNA expressions of oxidative stress molecules (gp91phox, iNOS, and Nrf2) and inflammation-related molecules (IL-1β, IL-6, TNFα, and TLR4) in the mouse hypothalamus, which can be alleviated by Iptakalim (Ipt), an ATP-sensitive potassium (K-ATP) channel opener (Zhao et al., 2017). The loss-of-function mutations of the aryl hydrocarbon receptor-interacting protein gene (AIP) are well-recognized in PAs (Hernández-Ramírez et al., 2018). The aryl hydrocarbon receptor signaling is also revealed by multiomics as an oxidative stress-related signaling pathway in PAs (Long et al., 2019). Further studies show that AIP interacts with antioxidants, chaperone and stress response-related proteins, and cytoskeletal proteins, including HSPA5, HSPA9, HSP90AA1, HSP90AB1, HSPA8, SOD1, TUBB, TUBB2A, and NME1; AIP variants show the impaired interaction of AIP with HSPA8, HSP90AB1, NME1, SOD1, TUBB, and TUBB2A; AIP-mutated PAs show the reduced expression of TUBB2A (Cuadrado et al., 2019). The levels of MnSOD and total antioxidant capability (TAC) are significantly decreased in GH-secreting PAs (Ilhan et al., 2018). The frequencies of micronuclei (MN), nucleoplasmic bridges, nuclear buds, apoptotic and necrotic cells, and plasma 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels in peripheral blood lymphocytes are significantly increased in PRL-secreting PAs, which indicates the increased oxidative damage in PRL-secreting PAs (Bitgen et al., 2016). Oxidative stress and mitochondrial dysfunction have been revealed by multiple proteomics and nitroproteomics studies in human PAs (Zhan and Desiderio, 2010a; Zhan et al., 2013; Zhan et al., 2014a; Zhan et al., 2014b; Wang X. et al., 2015; Long et al., 2019). Also, tumor inflammation is an important pathophysiological characteristic in human PAs, which is always tightly associated with oxidative stress and chronic inflammation. The relationship among age-related disease, chronic inflammation, and oxidative stress has also been discussed (Pizza et al., 2011; Liguori et al., 2018; Qian et al., 2019). Oxidative stress is also involved in the processes of anti-proliferative effect and cell death induced by dopamine in the pituitary tumor cells via dopamine D2 receptors through p38 MAPK, and ERK pathways (An et al., 2003). Therefore, oxidative stress and antioxidative stress response extensively exist in PA pathogenesis. Nrf2, as the core of oxidative stress response, could be the novel target used to develop effective therapeutic agents for human PAs (Kwak and Kensler, 2010; Furfaro et al., 2016; de la Vega et al., 2018).
FIGURE 1.
Nrf2-mediated oxidative stress response signaling pathways in human pituitary adenomas. AKR, Palmitoyltransferase; AKT, Protein kinase B; AOX4, Aldehyde oxidase 4; ARE, Antioxidant response element; ASK1, Apoptosis signal-regulating kinase 1; ATF4, Activating transcription factor 4; BACH1, Transcription regulator protein BACH1; CAT, catalase; CBP, CREB-binding protein; CBR4, carbonyl reductase 4; CCT7, T-complex protein 1 subunit eta; c-FOS, Proto-oncogene protein c-FOS; CLPP, Caseinolytic protease; Cul3, Cullin 3-based ubiquitin E3 ligase complex; Cyp, cytochrome P; EPHX1, Epoxide hydrolase 1; EpRE, Electrophile responsive element; ER, endoplasmic reticulum; ERK: Extracellular signal-related kinase; ERP29: endoplasmic reticulum protein 29; FKBP5, FK506-binding protein 5; FMO, Dimethylaniline monooxygenase [N-oxide-forming]; FRA1, Fos-related antigen 1; FTH1, Ferritin heavy polypeptide 1; FTL1, ferritin light polypeptide; GCLC, glutamate-cysteine ligase catalytic subunit; GCLM, glutamate-cysteine ligase modifier subunit; GPX's, Glutathione peroxidases; GSK3β, glycogen synthase kinase 3β; GSR, glutathione reductase; GST, glutathione S-transferase; HERPUD1, Homocysteine-responsive endoplasmic reticulum-resident ubiquitin-like domain member 1 protein; HIP2, Ubiquitin-conjugating enzyme E2 K; HO-1, heme oxygenase 1; HSP22/40/90, heat shock proteins 22, 40 and 90; JNK, Jun N-terminal kinase; Keap1, Kelch-like ECH-associated protein 1; Maf, Musculoaponeurotic fibrosarcoma; MAPK, Mitogen-activated protein kinase; MEK, Mitogen-activated protein kinase kinase (MAPKK); MEKK, Mitogen-activated protein kinase kinase kinase (MAPKKK); MRP1, multidrug-resistant protein-1; NQO1, NAD(P)H:quinine oxidoreductase 1; Nrf2, Nuclear factor erythroid 2 p45-related factor 2; PERK: the double-stranded RNA (PKR)-activated protein kinase-like eukaryotic initiation factor 2 kinase; PI3K, Phosphatidylinositol 3 kinase; PKC, protein kinase C; PPIB, Peptidyl-prolyl cis-trans isomerase B; PRDX1, peroxiredoxin 1; PSM: multiple subunits of the 20S proteasome; PTPLAD1, 3-hydroxyacyl-CoA dehydratase 3; c-Raf, RAF proto-oncogene serine/threonine-protein kinase; Ras, GTPase Ras; ROS, reactive oxygen species; SOD, Superoxide dismutase; SQSTM1, sequestosome-1 protein; SR-B1, Scavenger receptor class B member 1; STIP1, stress induced phosphoprotein 1; TAK1, TGF beta-Activated Kinase 1; TXN1: thioredoxin; TRXR1, thioredoxin reductase 1; UBB, Polyubiquitin-B; UB2R1, Ubiquitin-conjugating enzyme E2 R1; UGT, UDP glucuronosyl transferase; USP14, ubiquitin-specific peptidase 14; and VCP, valosin-containing protein. Modified from Zhan X et al. (2010) (Zhan and Desiderio, 2010a), copyright permission from BioMed Central publisher open-access article, copyright 2010; and modified from Long Y et al. (2019) (Long et al., 2019), copyright permission from Frontiersin publisher open-access article, copyright 2019.
Therapeutic Status Targeting Nrf2 Signaling Pathways in Cancers
Nrf2 signaling, as the heart of oxidative stress response, is extensively related to cancer pathogenesis, which has attracted tremendous attention as possible anticancer therapeutic target. Nrf2 signaling-based anticancer therapeutic studies have been extensively carried out in multiple cancers, including acute myeloid leukemia, gallbladder cancer, renal carcinoma, pancreatic cancer, melanoma, hepatocellular carcinoma, lung cancer, colon cancer, ovarian cancer, breast cancer, esophageal cancer, and glioblastoma (Table 1). i) In acute myeloid leukemia, studies found that Nrf2 activators [dimethyl fumarate (DMF), tert-butylhydroquinone, or carnosic acid] and vitamin D derivatives can cooperatively induce acute myeloid leukemia cell differentiation to inhibit leukemia progression in a xenograft mouse model via activating the Nrf2/ARE signaling pathway (Nachliely et al., 2019). Novel pyrazolyl hydroxamic acid derivative (4f) can inhibit Nrf2 activity to induce apoptosis of human acute myeloid leukemia cells (Zhang et al., 2017). ii) In gallbladder cancer, one study found that atypical protein kinase Cι (aPKCι) can promote gallbladder tumorigenesis and chemoresistance of anticancer agent gemcitabine by competing with Nrf2 for binding to Keap1, implying that inhibiting the aPKC1-Keap1-Nrf2 axis might overcome drug resistance for the treatment of gallbladder cancer (Tian et al., 2019). iii) In renal carcinoma, one study found that the natural product chitosan oligosaccharide (COS) can inhibit human renal carcinoma cell proliferation in vitro and in vivo by promoting the expressions of Nrf2 and Nrf2 target genes such as HO-1, the modifier subunit of glutamate cysteine ligase, solute carrier family 7 member 11, glucose-regulated protein 78, protein RNA-like endoplasmic reticulum kinase, and cytochrome C. (Zhai et al., 2019). iv) In pancreatic cancer, one study found that anticancer agent resveratrol enhances the sensitivity of pancreatic cancer cells to gemcitabine via suppressing NAF-1 (nutrient-deprivation autophagy factor-1) expression, inducing ROS accumulation, and activating Nrf2 signaling pathways (Cheng et al., 2018). v) In melanoma, the co-treatment of Nrf2 inhibitor (brusatol, BR) and UVA irradiation can effectively inhibit melanoma growth by regulating AKT-Nrf2 pathway (Wang et al., 2018). vi) In hepatocellular carcinoma, one study found that potential Nrf2 inhibitors can sensitize chemotherapy drugs in hepatocellular carcinoma (Tian et al., 2018). Cordycepin (CA) can activate the Nrf2/HO-1/NF-κB pathway for its anti-hepatocarcinoma effect in N-nitrosodiethylamine (NDEA)-induced mouse hepatocellular carcinomas (Zeng et al., 2017). The novel indazolo[3,2-b]quinazolinone (IQ) derivatives, IQ-7 and IQ-12, can induce apoptosis of human hepatoma cells Hep3B and inhibit the Nrf2/ARE signaling pathway in Hep3B cells, and IQ-7 is suggested as a degree of specificity against cancer cells (Zhang et al., 2016). Also, dibenzoylmethane (DBM) can protect against carbon tetrachloride (CCl4)-induced liver injury by activating Nrf2 signaling via JNK, AMPK, and calcium signaling (Cao et al., 2017). vii) In lung cancer, one study found that the potent anticancer agent isodeoxyelephantopin can induce protective autophagy in lung cancer cells via the Nrf2-p62-keap1 pathway (Wang et al., 2017). The Nrf2 activators, DMF and the synthetic oleanane triterpenoids, activate the Nrf2 pathway as well as regulate different subsets of Nrf2 target genes and Nrf2-independent genes in lung cancer (Chian et al., 2014; To et al., 2015). viii) In colon cancer, one study found that anticancer agent sulforaphane (SFN) can activate Nrf2 signaling to suppress human colon cancer (Johnson et al., 2017). Also, taxifolin (TAX) can induce antioxidant response pathway and enhance level of Nrf2 protein, and act as effective chemopreventive agent capable of modulating inflammation in colon cancer (Manigandan et al., 2015). ix) In ovarian cancer, one study found that Nrf2 can mediate the response of cancer cells to the anti-HER2 drugs, trastuzumab and pertuzumab, in ovarian cancer cells (Khalil et al., 2016). Also, activation of Nrf2 pathway in ovarian cancer seems to be related to Keap1 mutations within highly conserved domains of Keap1 gene and that Nrf2 may serve as an important therapeutic target for novel drugs capable of preventing or reversing resistance to chemotherapy in ovarian cancer (Konstantinopoulos et al., 2011). x) In breast cancer, Nrf2 serves as a key regulator in chemotherapeutic resistance under hypoxia through ROS-Nrf2-GCLC-GSH pathway and can be a potential treatment for hypoxia induced drug resistance in breast cancer cells (Song et al., 2011; Syu et al., 2016). xi) In esophageal cancer, C-28 methyl ester of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid (CDDO-Me) can protect the cells against oxidative stress via inhibition of ROS generation, while CDDO-Me at low micromolar concentrations induces apoptosis by increasing ROS and decreasing intracellular glutathione levels in esophageal squamous cancer cells (Wang Y. Y. et al., 2015). xii) In glioblastoma, there are many potent anti-cancer agents targeting Nrf2 signaling for chemotherapy and chemoresistance in glioblastoma (Zhu et al., 2014). xiii) In osteosarcoma, the bioengineered Nrf2-siRNA can effectively interfere with the Nrf2 signaling pathway to improve chemosensitivity of human cancer cells (Li et al., 2018). Moreover, the PIM (proviral integration site for moloney murine leukemia virus) kinase inhibitors can reduce Nrf2 signaling and increase ROS to kill hypoxic tumor cells such as prostate cancer cells (PC4-LN4), colon cancer cells (HCT-116), and breast cancer cells (MB-MDA-231 and MB-MDA-231-ARE-Luc) (Warfel et al., 2016). One study shows that proteasome biogenesis is dependent on the Nrf2 transcriptional factor, thus proteasome inhibitors have been actively developed as potential anticancer drugs (Albornoz et al., 2019). Gallic acid (GA), Z-ligustilide (LIG), and senkyunolide A (SA) can individually or cooperatively target Nrf2/ARE pathway to prevent cancer (Liu et al., 2018). Therefore, it can be said that Keap1-Nrf2 signaling pathways have different roles at different stages of cancer (Leinonen et al., 2014; Furfaro et al., 2016; de la Vega et al., 2018). Multiple Nrf2 or Keap1 inhibitors have been reported; and some of them are in the stages of pre- and clinical trial towards the Nrf2 signaling for cancers. For example, sulforaphane can target Nrf2 and the Nrf2 target genes NQO1 and GCLC to prevent oral cancer, and a preclinical trail has been performed to study its chemopreventive activity for oral cancer (Bauman et al., 2016). A single centre, single arm prospective phase II clinical trial has been performed for phytosome complex of curcumin targeting Nrf2 signaling as a the complementary therapy of gemcitabine on pancreatic cancer (Pastorelli et al., 2018). However, none of these Nrf2 or Keap1 inhibitors have currently entered into real clinical applications, which suggests that the sole inhibition of Nrf2 might not be sufficient for anticancer. A rational combination of Nrf2 inhibitors with other chemical agents would be a better strategy to treat cancers (Zhang et al., 2019).
TABLE 1.
Current research status of therapeutic potentials targeting Nrf2-mediated oxidative stress response signaling pathways in different cancers.
| Cancer type | Experimental model | Chemical reagents or potential drugs | Possible mechanisms | References |
|---|---|---|---|---|
| Acute myeloid leukemia | Acute myeloid leukemia cells in a xenograft mouse model | Nrf2 activators: dimethyl fumarate (DMF), tert-butylhydroquinone, or carnosic acid | Cooperate with vitamin D derivatives to induce acute myeloid leukemia cell differentiation to inhibit leukemia progression in a xenograft mouse model via activating the Nrf2/ARE signaling pathway | Nachliely et al. (2019) |
| Human acute myeloid leukemia cells | Novel pyrazolyl hydroxamic acid derivative (4f) | Inhibit Nrf2 activity to induce apoptosis of human acute myeloid leukemia cells | Zhang et al. (2019) | |
| Gallbladder cancer | Gallbladder cancer cells | The aPKCι inhitors, Nrf2 activators, or gemcitabine | Atypical protein kinase Cι (aPKCι) can promote gallbladder tumorigenesis and chemoresistance of anticancer agent gemcitabine by competing with Nrf2 for binding to Keap1, implying that inhibiting the aPKCι-Keap1-Nrf2 axis might overcome drug resistance for the gallbladder cancer treatment | Tian et al. (2019) |
| Renal carcinoma | Human renal carcinoma cells | Chitosan oligosaccharide (COS) | Inhibit human renal carcinoma cell proliferation in vitro and in vivo by promoting the expressions of Nrf2 and Nrf2 target genes such as HO-1, the modifier subunit of glutamate cysteine ligase, solute carrier family 7 member 11, glucose-regulated protein 78, protein RNA-like endoplasmic reticulum kinase, and cytochrome C,etc. | Zhai et al. (2019) |
| Pancreatic cancer | Pancreatic cancer cells | Resveratrol | Enhance the sensitivity of pancreatic cancer cells to gemcitabine via suppressing NAF-1 expression, inducing ROS accumulation, and activating Nrf2 signaling pathways | Cheng et al. (2018) |
| Melanoma | Melanoma cells | Nrf2 inhibitor: Brusatol (BR) | The co-treatment of brusatol and UVA irradiation can effectively inhibit melanoma growth by regulating the AKT-Nrf2 pathway | Wang et al. (2018) |
| Hepatocellular carcinoma | Hepatocellular carcinoma (HCC) cells | Vitamin C (VC), all-trans retinoic acid (ATRA), ochratoxin A (OTA), bexarotene, flavonoids (including brusatol, luteolin, apigenin and chrysin), ruthenium (Ru) metal complexes, ursolic acid (UA), halofuginone, trigonelline, quercetin, and isoniazid | Sensitize chemotherapy drugs in hepatocellular carcinoma | Tian et al. (2018) |
| Mouse hepatocellular carcinoma model | Cordycepin (CA) | Activate the Nrf2/HO-1/NF-κB pathway for its anti-hepatocarcinoma effect in N-nitrosodiethylamine (NDEA)-induced mouse hepatocellular carcinomas | Zeng et al. (2017) | |
| Hep3B (human hepatoma cell) and HL-7702 (normal human liver cell) cell lines | Novel indazolo[3,2-b] quinazolinone (IQ) derivatives: IQ-7 and IQ-12 | Induce apoptosis and inhibit the Nrf2/ARE signaling pathway in Hep3B cells, and IQ-7 was suggested a degree of specificity against cancer cells. | Zhang et al. (2016) | |
| Liver injury mouse model | Dibenzoylmethane (DBM) | Protect against carbon tetrachloride (CCl4)-induced liver injury by activating Nrf2 signaling via JNK, AMPK, and calcium signaling | Cao et al. (2017) | |
| Lung cancer | Lung cancer cells | The potent anticancer agent: Isodeoxyelephantopin | Induce protective autophagy in lung cancer cells via the Nrf2-p62-keap1 pathway | Wang et al. (2017) |
| RAW 264.7 mouse macrophage-like cells, in VC1 lung cancer cells, and in the A/J model of lung cancer | Two clinically relevant classes of Nrf2 activators: DMF, and the synthetic oleanane triterpenoids –C-28 methyl ester of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid (CDDO)-Imidazolide (CDDO-Im) and CDDO-Methyl ester (CDDO-Me) | Activate the Nrf2 pathway as well as regulate different subsets of Nrf2 target genes and Nrf2-independent genes | Chian et al. (2014) and To et al. (2015) | |
| Colon cancer | SFN-treated human colon cancer cells and non-transformed colonic epithelial cells | Anticancer agent: Sulforaphane (SFN) | Regulate the activity of antioxidant and the detoxification of carcinogens via Nrf2 signaling to suppress human colon cancer | Johnson et al. (2017) |
| 1, 2-dimethyl hydrazine (DMH)-induced mouse colon model | Taxifolin (TAX) | Induce antioxidant response pathway, enhance level of Nrf2 proteins, and act as effective chemopreventive agent capable of modulating inflammatory | Manigandan et al. (2015) | |
| Ovarian cancer | Human ovarian cancer cell lines: PEO4, OVCAR4, and SKOV3 | Anti-HER2 drugs: Trastuzumab and Pertuzumab | HER2 targeting by antibodies inhibited growth in association with persistent ROS generation, glutathione (GSH) depletion, reduction in NRF2 levels, and inhibition of NRF2 function in ovarian cancer cell lines | Khalil et al. (2016) |
| Human epithelial ovarian cancer (EOC) cell lines | Keap1 mutation reagent | Activation of Nrf2 pathway in EOC seems to be related to Keap1 mutations within highly conserved domains of the Keap1 gene; and Nrf2 may serve as an important therapeutic target for novel drugs capable of preventing or reversing resistance to chemotherapy in EOC | Konstantinopoulos et al. (2011) | |
| Breast cancer | Breast cancer cells, and mouse model | Target antioxidant enzymes: GCLC and GCLM | Nrf2 serves as a key regulator in chemotherapeutic resistance under hypoxia through ROS-Nrf2-GCLC-GSH pathway, and can be a potential treatment for hypoxia-induced drug resistance in breast cancer cells. | Syu et al. (2016) and Song et al. (2011) |
| Esophageal cancer | Esophageal squamous cancer cells (ESCC): Ec109 and KYSE70 cells | CDDO-Me | Protects the cells against oxidative stress via inhibition of ROS generation, while CDDO-Me at low micromolar concentrations induces apoptosis by increasing ROS and decreasing intracellular glutathione levels | Wang X. et al. (2015) |
| Glioblastoma | Glioblastoma cells | Potential anti-cancer agents | Targeting Nrf2 signaling for chemotherapy and chemoresistance | Zhu et al. (2014) |
| Osteosarcoma | Human osteosarcoma 143B and MG63 cells | The bioengineered Nrf2-siRNA | Interfere with the Nrf2 signaling pathway to reduce the expression of NRF2-regulated oxidative enzymes and lead to higher intracellular ROS levels; knocking down NRF2 with bioengineered siRNA agent improves chemosensitivity of cancer cells, which is related to the suppression of NRF2-regulated efflux ABC transporters. | Li et al. (2018) |
| Other cancers | prostate cancer cell PC4-LN4; colon cancer cell HCT-116; breast cancer cells MB-MDA-231 and MB-MDA-231-ARE-Luc | PIM kinases inhibitors | Inhibit Nrf2 signaling and increase ROS to kill hypoxic tumor cells in a HIF-1-independent manner by controlling its cellular localization | Warfel et al. (2016) |
| Mammalian cancer cells | Proteasome inhibitors | In response to proteasome inhibition, several responses are activated, such as the ALP, proteaphagy, the transcriptional upregulation of the autophagy Ubreceptor p62/SQSTM1, and proteasome genes, by Nrf1 and Nrf1/Nrf2 transcription factors, respectively. | Albornoz et al. (2019) | |
| Mouse epidermal cells (JB6 P+), | Gallic acid (GA), Z-ligustilide (LIG), and senkyunolide A (SA) | GA, LIG, and SA in Si-Wu-Tang (SWT) can individually or cooperatively target the Nrf2/ARE pathway to prevent cancer. | Liu et al. (2018) |
ALP, Autophagic-Lysosomal Pathway; ATRA, All-trans retinoic acid; BR, Brusatol; CA, Cordycepin; CDDO, C-28 methyl ester of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid; COS, Chitosan oligosaccharide; DBM, Dibenzoylmethane; DMF, dimethyl fumarate; GA, Gallic acid; IQ, Indazolo[3,2-b] quinazolinone; LIG, Z-ligustilide; OTA, Ochratoxin A; PIM, The Proviral Integration site for Moloney murine leukemia virus; Ru, Ruthenium; SA, Senkyunolide A; SFN, Sulforaphane; TAX, Taxifolin; UA, Ursolic acid; VC, vitamin C.
Potential of Targeting Nrf2 Signaling as New Therapeutic Strategy for PAs
As described above, many omics studies in human PA tissues and experimental studies in PA cells and animal models demonstrate that oxidative stress and oxidative damage is the important hallmark of PA pathogenesis. Nrf2-mediated oxidative stress response signaling pathways are at the heart of oxidative stress response, and many chemical agents targeting Nrf2 signaling pathways have been developed and tested as potential anticancer drugs for different cancers. This clearly demonstrates the potential of targeting Nrf2 signaling pathways as new therapeutic strategies for PAs. However, the use of Nrf2 signaling as a therapeutic target for PAs has not been studied. We strongly believe that the Nrf2-mediated oxidative stress response signaling pathways are the promising targets for novel therapeutic strategies for PAs. Furthermore, MAPK signaling pathways including ERK, JNK, and p38 MAPK clearly regulate Nrf2 signaling (Figure 1). Moreover, MAPK signaling pathways have been recognized as potential therapeutic targets for PAs (Lu et al., 2019). The combined use of Nrf2 inhibitors targeting Nrf2 signaling and ERK inhibitors [e.g., somatostatin analogs pasireotide (SOM230) and octreotide (OCT), or dopamine] plus p38 activators (e.g., cabergoline, bromocriptine, and fulvestrant) or JNK activators (e.g., ursolic acid, UA) targeting MAPK signaling pathways (Lu et al., 2019) might produce better anti-tumor effects on PAs. In addition, oxidative stress is tightly associated with mitochondrial dysfunctions, both operate in PAs (Zhan and Desiderio, 2010a; Li and Zhan, 2019; Long et al., 2019). Some drugs targeting mitochondria are also recognized as a therapeutic strategy for PAs, including pyrimethamine, temozolomide, melatonin, melatonin inhibitors, gossypol acetate, 18 beta-glycyrrhetinic acid, T-2 toxin, Yougui pill, cyclosporine A, grifolic acid, paeoniflorin, and dopamine agonists (Li and Zhan, 2019). Therefore, the combined use of Nrf2 inhibitors targeting Nrf2 signaling and drugs targeting metochondria could be another way to generate better anti-tumor effects on PAs.
Conclusion
Pituitary adenoma (PA) is a common and important disease that occurs in the hypothalamic-pituitary-target organ axis system and seriously affects human endocrine system and health. The imbalance between oxidative stress and the antioxidant defense system is an important pathophysiological characteristic in PAs, which has been evidenced by many omics analysis in PA tissues and experimental studies in PA cells and animal models. Nrf2 signaling is at the heart of oxidative stress response signaling pathways. Multiple anticancer agents targeting Nrf2-mediated oxidative stress response pathways have been developed and tested as potential therapeutic drugs for different cancers. However, Nrf2 signaling and targeting Nrf2 signaling as a therapeutic strategy has not yet been extensively studied in PAs. We strongly recommend the emphasis on in-depth studies of Nrf2 signaling and potential therapeutic agents targeting Nrf2 signaling pathways in PAs. Furthermore, the combined use of Nrf2 inhibitors targeting Nrf2 signaling and ERK inhibitors plus p38 activators or JNK activators targeting MAPK signaling pathways, or drugs targeting mitochondria dysfunction pathway might produce better anti-tumor effects on PAs.
Acknowledgments
The authors acknowledge the financial supports from the Shandong First Medical University Talent Introduction Funds (to XZ), and the Hunan Provincial Hundred Talent Plan (to XZ). We acknowledge that Xiaohan Zhan from Wesleyan University in United States critically revised the manuscript.
Author Contributions
XZ conceived the concept, collected and analyzed literature, designed, coordinated, wrote and revised manuscript, and was responsible for its financial supports and the corresponding works. JL and TZ participated in literature collection and analysis, and prepared figures. All authors approved the final manuscript.
Funding
The financial supports from the Shandong First Medical University Talent Introduction Funds (to XZ), and the Hunan Provincial Hundred Talent Plan (to XZ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
Glossary
- ACTH
Adrenocorticotropin
- AFAR
aldo-keto reductase family 7 member A2
- AIP
Aryl hydrocarbon receptor interacting protein gene
- AKR
Palmitoyltransferase AKT
- AKR1B1
aldo-keto reductase family 1 member B
- AKT
Protein kinase B
- AOX4
Aldehyde oxidase 4
- AP-1
Activator protein-1
- aPKCι
Atypical protein kinase Cι
- ARE
Antioxidant response element
- ARS
Acute restraint stress
- ASK1
Apoptosis signal-regulating kinase 1
- ATF4
Activating transcription factor 4
- ATP5A1
ATP synthase subunit alpha, mitochondrial
- ATP5B
ATP synthase, H+ transporting mitochondrial F1 complex, beta subunit
- BACH1
Transcription regulator protein BACH1
- Bax
BCL2 associated X, apoptosis regulator
- BCL2
BCL2 apoptosis regulator
- BR
Brusatol
- CALM
calmodulin
- CAT
catalase
- CBP
CREB-binding protein
- CBR4
carbonyl reductase 4
- CCL2
C-C motif chemokine ligand 2
- CCT7
T-complex protein 1 subunit eta
- c-FOS
Proto-oncogene protein c-FOS
- CLPP
Caseinolytic protease
- COS
Chitosan oligosaccharide
- COX6B
cytochrome c oxidase subunit 6B
- c-MAF
(MAF) MAF bZIP transcription factor
- c-Raf
RAF proto-oncogene serine/threonine-protein kinase
- CRS
Chronic restraint stress
- Cul3
Cullin 3-based ubiquitin E3 ligase complex
- Cyp
cytochrome P
- CYP1A
cytochrome P450 family 1 subfamily A
- CYP2A
cytochrome P450 family 2 subfamily A
- CYP2C
cytochrome P450 family 2 subfamily C
- CYP3A
cytochrome P450 family 3 subfamily A
- CYP4A
cytochrome P450 family 4 subfamily A
- DBM
Dibenzoylmethane
- DEG
Differentially expressed gene
- DEP
Differentially expressed protein
- DMF
Dimethyl fumarate
- EPHX1
Epoxide hydrolase 1
- EpRE
Electrophile responsive element
- ER
endoplasmic reticulum
- ERK
Extracellular signal-related kinase
- ERK1/2
mitogen-activated protein kinase
- ERK5
mitogen-activated protein kinase
- ERP29
endoplasmic reticulum protein 29
- ESR1
estrogen receptor 1
- ESR2
estrogen receptor 2
- FKBP5
FK506-binding protein 5
- FMO
Dimethylaniline monooxygenase [N-oxide-forming]
- FPA
Functional pituitary adenoma
- FRA1
Fos-related antigen 1
- FSH
Follicle-stimulating hormone
- FSHRH
Follicle-stimulating hormone-releasing hormone
- FTH1
Ferritin heavy polypeptide 1
- FTL1
ferritin light polypeptide
- GA
Gallic acid
- GCLC
glutamate-cysteine ligase catalytic subunit
- GCLM
glutamate-cysteine ligase modifier subunit
- GH
Growth hormone
- GPX4
glutathione peroxidase 4
- GPX's
Glutathione peroxidases
- GRP94
94 kD glucose-regulated protein
- GSK3β
glycogen synthase kinase 3β
- GSR
glutathione reductase
- GST
glutathione S-transferase
- GSTM2
glutathione S-transferase mu 2
- HERPUD1
Homocysteine-responsive endoplasmic reticulum-resident ubiquitin-like domain member 1 protein
- HIP2
Ubiquitin-conjugating enzyme E2 K
- HO-1
heme oxygenase 1
- HSP22/40/90
heat shock proteins 22, 40 and 90
- HSP27
heat shock protein 27
- HSP70
(HSPA4) heat shock protein family A member 4
- HSP90
heat shock protein 90
- HSP90AA1
heat shock protein 90 alpha family class A member 1
- HSP90AB1
heat shock protein 90 alpha family class B member 1
- HSP94
heat shock protein 94
- HSPA5
heat shock protein family A (Hsp70) member 5
- HSPA8
heat shock protein family A (Hsp70) member 8
- HSPA9
heat shock protein family A (Hsp70) member 9
- HSPCA
(HSP90AA1) heat shock protein 90 alpha family class A member 1
- HSPCB
(HSP90AB1) heat shock protein 90 alpha family class B member 1
- IL-1β
interleukin 1 beta
- IL-6
interleukin 6
- iNOS
Inducible nitric synthase
- IP3R
(ITPR1) inositol 1,4,5-trisphosphate receptor type 1
- Ipt
Iptakalim
- JNK
Jun N-terminal kinase
- JNK1
(MAPK8) mitogen-activated protein kinase 8
- JNK 2
(MAPK9) mitogen-activated protein kinase 9
- JUN
Jun proto-oncogene, AP-1 transcription factor subunit
- K-ATP
ATP-sensitive potassium
- Keap1
Kelch-like ECH-associated protein 1
- LH
Luteinizing hormone
- LHRH
Luteinizing hormone-releasing hormone
- LIG
Z-ligustilide
- Maf
Musculoaponeurotic fibrosarcoma
- MAPKs
Mitogen-activated protein kinases
- MEK
Mitogen-activated protein kinase kinase (MAPKK)
- MEKK
Mitogen-activated protein kinase kinase kinase (MAPKKK)
- MN
Micronucleus
- MRP1
multidrug-resistant protein-1
- NAC
N-acetylcysteine
- NAF-1
Nutrient-deprivation autophagy factor-1
- NDEA
N-nitrosodiethylamine
- NDUFS8
(NADH) ubiquinone oxidoreductase core subunit S8
- NF-kB
Nuclear factor kB
- NFPA
Nonfunctional pituitary adenoma
- NME1
NME/NM23 nucleoside diphosphate kinase 1
- NO
Nitric oxide
- NQO1
NAD(P)H:quinine oxidoreductase 1
- Nrf2
Nuclear factor erythroid 2 p45-related factor 2
- Nur77
(NR4A1) nuclear receptor subfamily 4 group A member 1
- O2.-
Superoxide radical
- 8-OHdG
8-hydroxy-2’-deoxyguanosine
- OCT
octreotide
- OH
hydroxyl radical
- ONOO-
Peroxynitrite anion
- PA
Pituitary adenoma
- PACAP38
Pituitary adenylate cyclase-activating polypeptide 38
- PDK1
pyruvate dehydrogenase kinase 1
- PERK
the double-stranded RNA (PKR)-activated protein kinase-like eukaryotic initiation factor 2 kinase
- PFK
phosphofructokinase
- PI3K
Phosphatidylinositol 3 kinase
- PINK1
PTEN-induced putative kinase protein 1
- PKA
cAMP dependent protein kinase
- PKC
protein kinase C
- POMC
proopiomelanocortin
- PPIB
Peptidyl-prolyl cis-trans isomerase B
- PP2C
putative protein phosphatase
- PRDX1
peroxiredoxin 1
- PRL
Prolactin
- PSM
multiple subunits of the 20S proteasome
- Pter
Pterostilbene
- PTPLAD1
3-hydroxyacyl-CoA dehydratase 3
- Ras
GTPase Ras
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
- SA
Senkyunolide A
- SFN
Sulforaphane
- SOD
Superoxide dismutase
- SOD1
superoxide dismutase 1
- SOM230
somatostatin analogs pasireotide
- SQSTM1
sequestosome-1 protein
- SR-B1
Scavenger receptor class B member 1
- STIP1
stress induced phosphoprotein 1
- TAC
Total antioxidant capability
- TAK1
TGF beta-Activated Kinase 1
- TGM2
transglutaminase 2
- TLR4
toll like receptor 4
- TNFα
tumor necrosis factor alpha
- TRXR1
thioredoxin reductase 1
- TUBB
tubulin beta class I
- TUBB2A
tubulin beta 2A class IIa
- TXN1
thioredoxin
- UBB
Polyubiquitin-B
- UB2R1
Ubiquitin-conjugating enzyme E2 R1
- UGT
UDP glucuronosyl transferase
- USP14
ubiquitin-specific peptidase 14
- UV
ultraviolet
- VCP
valosin-containing protein
References
- Afolabi O. B., Oloyede O. I., Agunbiade S. O. (2018). Inhibitory potentials of phenolic-rich extracts from Bridelia ferruginea on two key carbohydrate-metabolizing enzymes and Fe2+-induced pancreatic oxidative stress. J. Integrat Med. 16, 192–198. 10.1016/j.joim.2018.04.006 [DOI] [PubMed] [Google Scholar]
- Albornoz N., Bustamante H., Soza A., Burgos P. (2019). Cellular responses to proteasome inhibition: molecular mechanisms and beyond. Int. J. Mol. Sci. 20 (14), E3379. 10.3390/ijms20143379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J. J., Cho S. R., Jeong D. W., Park K. W., Ahn Y. S., Baik J. H. (2003). Anti-proliferative effects and cell death mediated by two isoforms of dopamine D2 receptors in pituitary tumor cells. Mol. Cell Endocrinol. 206 (1-2), 49–62. 10.1016/s0303-7207(03)00236-3 [DOI] [PubMed] [Google Scholar]
- Baird L., Yamamoto M. (2020). The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell Biol. 40 (13), e00099. 10.1128/MCB.00099-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman J. E., Zang Y., Sen M., Li C., Wang L., Egner P. A., et al. (2016). Prevention of carcinogen-induced oral cancer by sulforaphane. Cancer Prev. Res. 9 (7), 547–557. 10.1158/1940-6207.CAPR-15-0290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellezza I., Giambanco I., Minelli A., Donato R., Bellezza I., et al. (2018). Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cel. Res. 1865 (5), 721–733. 10.1016/j.bbamcr.2018.02.010 [DOI] [PubMed] [Google Scholar]
- Benlloch M., Obrador E., Valles S. L., Rodriguez M. L., Sirerol J. A., Alcácer J., et al. (2016). Pterostilbene decreases the antioxidant defenses of aggressive cancer cells in vivo: a physiological glucocorticoids- and Nrf2-dependent mechanism. Antioxid. Redox Signal 24 (17), 974–990. 10.1089/ars.2015.6437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beranova-Giorgianni S., Giorgianni F., Desiderio D. M. (2002). Analysis of the proteome in the human pituitary. Proteomics 2, 534–542. [DOI] [PubMed] [Google Scholar]
- Beranova-Giorgianni S., Zhao Y., Desiderio D. M., Giorgianni F. (2006). Phosphoproteomic analysis of the human pituitary. Pituitary 9, 109–120. 10.1007/s11102-006-8916-x [DOI] [PubMed] [Google Scholar]
- Bitgen N., Donmez-Altuntas H., Bayram F., Cakir I., Hamurcu Z., Diri H., et al. (2016). Increased micronucleus, nucleoplasmic bridge, nuclear bud frequency and oxidative DNA damage associated with prolactin levels and pituitary adenoma diameters in patients with prolactinoma. Biotech. Histochem. 91 (2), 128–136. 10.3109/10520295.2015.1101163 [DOI] [PubMed] [Google Scholar]
- Bocca L., Valenti S., Cuttica C. M., Spaziante R., Giordano G., Giusti M. (2000). Nitric oxide biphasically modulates GH secretion in cultured cells of GH-secreting human pituitary adenomas. Minerva. Endocrinol. 25 (3-4), 55–59. [PubMed] [Google Scholar]
- Cao M., Wang H., Guo L., Yang S., Liu C., Khor T. O., et al. (2017). Dibenzoylmethane protects against CCl4-induced acute liver injury by activating Nrf2 via JNK, AMPK, and calcium signaling. AAPS J. 19 (6), 1703–1714. 10.1208/s12248-017-0133-1 [DOI] [PubMed] [Google Scholar]
- Ceccatelli S., Hulting A. L., Zhang X., Gustafsson L., Villar M., Hökfelt T. (1993). Nitric oxide synthase in the rat anterior pituitary gland and the role of nitric oxide in regulation of LH secretion. Proc. Natl. Acad. Sci. U.S.A. 90 (23), 11292–11296. 10.1073/pnas.90.23.11292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q. M., Maltagliati A. J. (2018). Nrf2 at the heart of oxidative stress and cardiac protection. Physiol. Genomics 50 (2), 77–97. 10.1152/physiolgenomics.00041.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Yan B., Chen K., Jiang Z., Zhou C., Cao J., et al. (2018). Resveratrol-induced downregulation of NAF-1 enhances the sensitivity of pancreatic cancer cells to gemcitabine via the ROS/Nrf2 signaling pathways. Oxid Med. Cell Longev. 2018, 9482018. 10.1155/2018/9482018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T., Wang Y., Lu M., Zhan X., Zhou T., Li B., et al. (2019). Quantitative analysis of proteome in non-functional pituitary adenomas: clinical relevance and potential benefits for the patients. Front. Endocrinol. 10, 854. 10.3389/fendo.2019.00854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T., Zhan X. (2017). Pattern recognition for predictive, preventive, and personalized medicine in cancer. EPMA J. 8, 51–60. 10.1007/s13167-017-0083-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chian S., Thapa R., Chi Z., Wang X. J., Tang X. (2014). Luteolin inhibits the Nrf2 signaling pathway and tumor growth in vivo . Biochem. Biophys. Res. Commun. 447 (4), 602–608. 10.1016/j.bbrc.2014.04.039 [DOI] [PubMed] [Google Scholar]
- Chu F. F., Esworthy R. S., Chu P. G., Longmate J. A., Huycke M. M., Wilczynski S., et al. (2004). Bacteria-induced intestinal cancer in mice with disrupted GPx1 and Gpx2 genes. Cancer Res. 64 (3), 962–968. 10.1158/0008-5472.can-03-2272 [DOI] [PubMed] [Google Scholar]
- Cloer E. W., Goldfarb D., Schrank T. P., Weissman B. E., Major M. B. (2019). NRF2 activation in cancer: from DNA to protein. Cancer Res. 79 (5), 889–898. 10.1158/0008-5472.CAN-18-2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado A., Rojo A. I., Wells G., Hayes J. D., Cousin S. P., Rumsey W. L., et al. (2019). Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 18 (4), 295–317. 10.1038/s41573-018-0008-x [DOI] [PubMed] [Google Scholar]
- Cuttica C. M., Giusti M., Bocca L., Sessarego P., De Martini D., Valenti S., et al. (1997). Nitric oxide modulates in vivo and in vitro growth hormone release in acromegaly. Neuroendocrinology 66 (6), 426–431. 10.1159/000127268 [DOI] [PubMed] [Google Scholar]
- Dayalan Naidu S., Dinkova-Kostova A. T. (2020). KEAP1, a cysteine-based sensor and a drug target for the prevention and treatment of chronic disease. Open Biol. 10 (6), 200105. 10.1098/rsob.200105.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Vega M. R., Chapman E., Zhang D. D. (2018). NRF2 and the hallmarks of cancer. Cancer Cell 34 (1), 21–43. 10.1016/j.ccell.2018.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyu H., Luqing C., Xianglian L., Pu G., Qirong L., Xu W., et al. (2018). Protective mechanisms involving enhanced mitochondrial functions and mitophagy against T-2 toxin-induced toxicities in GH3 cells. Toxicol. Lett. 295, 41–53. 10.1016/j.toxlet.2018.05.041 [DOI] [PubMed] [Google Scholar]
- Dhakshinamoorthy S., Porter A. G. (2004). Nitric oxide-induced transcriptional up-regulation of protective genes by Nrf2 via the antioxidant response element counteracts apoptosis of neuroblastoma cells. J. Biol. Chem. 279 (19), 20096–20107. 10.1074/jbc.M312492200 [DOI] [PubMed] [Google Scholar]
- Duvilanski B. H., Zambruno C., Seilicovich A., Pisera D., Lasaga M., Diaz M. C., et al. (1995). Role of nitric oxide in control of prolactin release by the adenohypophysis. Proc. Natl. Acad. Sci. U.S.A. 92 (1), 170–174. 10.1073/pnas.92.1.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebokaiwe A. P., Obeten K. E., Okori S. O., David E. E., Olusanya O., Chukwu C. J., et al. (2020). Co-administration of selenium nanoparticles and metformin abrogate testicular oxidative injury by suppressing redox imbalance, augmenting sperm quality and Nrf2 protein expression in streptozotocin-induced diabetic rats. Biol. Trace Elem. Res. 198, 544. 10.1007/s12011-020-02082-2 [DOI] [PubMed] [Google Scholar]
- Elchuri S., Oberley T. D., Qi W., Eisenstein R. S., Jackson Roberts L., et al. (2005). CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene 24 (3), 367–380. 10.1038/sj.onc.1208207 [DOI] [PubMed] [Google Scholar]
- Evans C. O., Moreno C. S., Zhan X., Mccabe M. T., Vertino P. M., Desiderio D. M., et al. (2008). Molecular pathogenesios f human prolactinomasi dentified by genee xpressionp rofiling, RT-qPCR, and proteomic analyses. Pituitlry 11, 231–245. 10.1007/s11102-007-0082-2 [DOI] [PubMed] [Google Scholar]
- Feng J., Zhang Q., Zhou Y., Yu S., Hong L., Zhao S., et al. (2018). Integration of proteomics and metabolomics revealed metabolite-protein networks in ACTH-secreting pituitary adenoma. Front. Endocrinol. 9, 678. 10.3389/fendo.2018.00678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furfaro A. L., Traverso N., Domenicotti C., Piras S., Moretta L., Marinari U. M., et al. (2016). The Nrf2/HO-1 axis in cancer cell growth and chemoresistance. Oxid Med. Cell 2016, 1958174. 10.1155/2016/1958174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland F., Lacroix L., Saulnier P., Dessen P., Meduri G., Bernier M., et al. (2010). Differential gene expression profiles of invasive and non-invasive non-functioning pituitary adenomas based on microarray analysis. Endocr. Relat. Cancer 17, 361–371. 10.1677/ERC-10-0018 [DOI] [PubMed] [Google Scholar]
- Giorgianni F., Beranova-Giorgianni S., Desiderio D. M. (2004). Identification and characterization of phosphorylated proteins in the human pituitary. Proteomics 4, 587–598. 10.1002/pmic.200300584 [DOI] [PubMed] [Google Scholar]
- Giorgianni F., Desiderio D. M., Beranova-Giorgianni S. (2003). Proteome analysis using isoelectric focusing in immobilized pH gradient gels followed by mass spectrometry. Electrophoresis 24, 253–259. 10.1002/elps.200390021 [DOI] [PubMed] [Google Scholar]
- Gonçalves Á. d. C., Moreira E. J. S., Portari G. V. (2019). Benfotiamine supplementation prevents oxidative stress in anterior tibialis muscle and heart. J. Integr. Med. 17, 423–429. 10.1016/j.joim.2019.07.001 [DOI] [PubMed] [Google Scholar]
- Grech G., Zhan X., Yoo B. C., Bubnov R., Hagan S., Danesi R., et al. (2015). EPMA position paper in cancer: current overview and future perspectives. EPMA J. 6, 9. 10.1186/s13167-015-0030-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambright H. G., Meng P., Kumar A. P., Ghosh R. (2015). Inhibition of PI3K/AKT/mTOR axis disrupts oxidative stress-mediated survival of melanoma cells. Oncotarget 6 (9), 7195–7208. 10.18632/oncotarget.3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris I. S., Treloar A. E., Inoue S., Sasaki M., Gorrini C., Lee K. C., et al. (2015). Glutathione and thioredoxin antioxidant pathways synergize to drive cancerinitiation and progression. Cancer Cell 27 (2), 211–222. 10.1016/j.ccell.2014.11.019 [DOI] [PubMed] [Google Scholar]
- Hernández-Ramírez L. C., Morgan R. M. L., Barry S., D'Acquisto F., Prodromou C., Korbonits M. (2018). Multi-chaperone function modulation and association with cytoskeletal proteins are key features of the function of AIP in the pituitary gland. Oncotarget 9 (10), 9177–9198. 10.18632/oncotarget.24183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetland G., Tangen J.-M., Mahmood F., Mirlashari M. R., Nissen-Meyer L. S. H., Nentwich I., et al. (2020). Antitumor, anti-Inflammatory and antiallergic effects of Agaricus blazei Mushroom extract and the related medicinal basidiomycetes mushrooms, hericium erinaceus and Grifola frondosa: a Review of Preclinical and Clinical Studies. Nutrients 12 (5), 1339. 10.3390/nu12051339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R., Wang X., Zhan X. (2013). Multi-parameter systematic strategies for predictive, preventive and personalised medicine in cancer. EPMA J. 4, 2. 10.1186/1878-5085-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. S., Chen H. W., Lin T. Y., Lin A. H., Lii C. K. (2018). Shikonin upregulates the expression of drug-metabolizing enzymes and drug transporters in primary rat hepatocytes. J. Ethnopharmacol. 216, 18–25. 10.1016/j.jep.2018.01.026 [DOI] [PubMed] [Google Scholar]
- Hybertson B. M., Gao B., Bose S. K., McCord J. M. (2011). Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol. Aspects Med. 32 (4-6), 234–246. 10.1016/j.mam.2011.10.006 [DOI] [PubMed] [Google Scholar]
- Ilhan M., Turgut S., Turan S., Demirci Cekic S., Ergen H. A., Korkmaz Dursun G., et al. (2018). The assessment of total antioxidant capacity and superoxide dismutase levels, and the possible role of manganese superoxide dismutase polymorphism in acromegaly. Endocr. J. 65 (1), 91–99. 10.1507/endocrj.EJ17-0300 [DOI] [PubMed] [Google Scholar]
- Johnson G. S., Li J., Beaver L. M., Dashwood W. M., Sun D., Rajendran P., et al. (2017). A functional pseudogene, NMRAL2P, is regulated by Nrf2 and serves as a coactivator of NQO1 in sulforaphane-treated colon cancer cells. Mol. Nutr. Food Res. 61 (4). 10.1002/mnfr.201600769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S. W., Rhee S. G., Chang T. S., Jeong W., Choi M. H. (2005). 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends Mol. Med. 11 (12), 571–578. 10.1016/j.molmed.2005.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil H. S., Langdon S. P., Goltsov A., Soininen T., Harrison D. J., Bown J., et al. (2016). A novel mechanism of action of HER2 targeted immunotherapy is explained by inhibition of NRF2 function in ovarian cancer cells. Oncotarget 7 (46), 75874–75901. 10.18632/oncotarget.12425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaunig J. E. (2018). Oxidative stress and cancer. Curr. Pharm. Des. 24 (40), 4771–4778. 10.2174/1381612825666190215121712 [DOI] [PubMed] [Google Scholar]
- Konstantinopoulos P. A., Spentzos D., Fountzilas E., Francoeur N., Sanisetty S., Grammatikos A. P., et al. (2011). Keap1 mutations and Nrf2 pathway activation in epithelial ovarian cancer. Cancer Res. 71 (15), 5081–5089. 10.1158/0008-5472.CAN-10-4668 [DOI] [PubMed] [Google Scholar]
- Kruk J., Aboul-Enein H. Y. (2017). Reactive oxygen and nitrogen species in carcinogenesis: implications of oxidative stress on the progression and development of several cancer types. Mini Rev. Med. Chem. 17 (11), 904–919. 10.2174/1389557517666170228115324 [DOI] [PubMed] [Google Scholar]
- Kruse A., Broholm H., Rubin I., Schmidt K., Lauritzen M. (2002). Nitric oxide synthase activity in human pituitary adenomas. Acta Neurol. Scand. 106 (6), 361–366. 10.1034/j.1600-0404.2002.01138.x [DOI] [PubMed] [Google Scholar]
- Kudryavtseva A. V., Krasnov G. S., Dmitriev A. A., Alekseev B. Y., Kardymon O. L., Sadritdinova A. F., et al. (2016). Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 7 (29), 44879–44905. 10.18632/oncotarget.9821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurisaka M., Nakajo T., Mori K. (2004). Immunohistochemical study on the expression of copper and zinc-superoxide dismutase (Cu, Zn-SOD) in human adenohypophysis and pituitary adenomas. No To Shinkei 46 (10), 948–954. [PubMed] [Google Scholar]
- Kwak M. K., Kensler T. W. (2010). Targeting NRF2 signaling for cancer chemoprevention. Toxicol. Appl. Pharmacol. 244 (1), 66–76. 10.1016/j.taap.2009.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen H. M., Kansanen E., Pölönen P., Heinäniemi M., Levonen A. L. (2014). Role of the Keap1-Nrf2 pathway in cancer. Adv. Cancer Res. 122, 281–320. 10.1016/B978-0-12-420117-0.00008-6 [DOI] [PubMed] [Google Scholar]
- Li H., Cao L., Yi P. Q., Xu C., Su J., Chen P. Z., et al. (2019). Pituitary adenylate cyclase-activating polypeptide ameliorates radiation-induced cardiac injury. Am. J. Transl. Res. 11 (10), 6585–6599. www.ajtr.org/ISSN:1943-8141/AJTR0102651. [PMC free article] [PubMed] [Google Scholar]
- Li N., Zhan X. (2019). Mitochondrial dysfunction pathway networks and mitochondrial dynamics in the pathogenesis of pituitary adenomas. Front. Endocrinol. 10, 690. 10.3389/fendo.2019.00690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P. C., Tu M. J., Ho P. Y., Jilek J. L., Duan Z., et al. (2018). Bioengineered NRF2-siRNA is effective to interfere with NRF2 pathways and improve chemosensitivity of human cancer cells. Drug Metab. Dispos. 46 (1), 2–10. 10.1124/dmd.117.078741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Dong S., Chen Q., Chen C., Dong Z. (2020). Selenium may suppress peripheral blood mononuclear cell apoptosis by modulating HSP70 and regulate levels of SIRT1 through reproductive hormone secretion and oxidant stress in women suffering fluorosis. Eur. J. Pharmacol. 878, 173098. 10.1016/j.ejphar.2020.173098 [DOI] [PubMed] [Google Scholar]
- Li W., Kong A. N. (2009). Molecular mechanisms of Nrf2-mediated antioxidant response. Mol. Carcinog. 48 (2), 91–104. 10.1002/mc.20465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Huang T. T., Carlson E. J., Melov S., Ursell P. C., Olson J. L., et al. (1995). Dilated cardiomyopathy and neonatal lethality in mutant mice lacking mangese superoxide dismutase. Nat. Genet. 11 (4), 376–381. 10.1038/ng1295-376 [DOI] [PubMed] [Google Scholar]
- Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., et al. (2018). Oxidative stress, aging, and diseases. Clin. Interv. Aging 13, 757–772. 10.2147/CIA.S158513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. M., Huang K. M., Qian L., Chatterjee P., Zhang S., Li R., et al. (2018). Effects of bioactive constituents in the Traditional Chinese Medicinal formula Si-Wu-Tang on Nrf2 signaling and neoplastic cellular transformation. Phytomedicine 40, 1–9. 10.1016/j.phymed.2017.12.031 [DOI] [PubMed] [Google Scholar]
- Liu P., Tian W., Tao S., Tillotson J., Wijeratne E. M. K., Gunatilaka A. A. L., et al. (2019). Non-covalent NRF2 activation confers greater cellular protection than covalent activation. Cell Chem Biol. 26 (10), 1427–1435. 10.1016/j.chembiol.2019.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. V., Jin L., Qian X., Zhang S., Scheithauer B. W. (1995). Nitric oxide synthase in the human pituitary gland. Am. J. Pathol. 146 (1), 86–94. [PMC free article] [PubMed] [Google Scholar]
- Long Y., Lu M., Cheng T., Zhan X., Zhan X. (2019). Multiomics-based signaling pathway network alterations in human non-functional pituitary adenomas. Front. Endocrinol. 10, 835. 10.3389/fendo.2019.00835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes M. B. S. (2017). The 2017 World Health Organization classification of tumors of the pituitary gland: a summary. Acta Neuropathol. 134, 521–535. 10.1007/s00401-017-1769-8 [DOI] [PubMed] [Google Scholar]
- Lu M., Wang Y., Zhan X. (2019). The MAPK pathway-based drug therapeutic targets in pituitary adenomas. Front. Endocrinol. 10, 330. 10.3389/fendo.2019.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Zhan X. (2018). The crucial role of multiomic approach in cancer research and clinically relevant outcomes. EPMA J. 9, 77–102. 10.1007/s13167-018-0128-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M. C., Ji J. A., Jiang Z. Y., You Q. D. (2016). The Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target: an update. Med. Res. Rev. 36 (5), 924–963. 10.1002/med.21396 [DOI] [PubMed] [Google Scholar]
- Lubelska K., Wiktorska K., Mielczarek L., Milczarek M., Zbroińska-Bregisz I., Chilmonczyk Z. (2016). Sulforaphane regulates NFE2L2/Nrf2-Dependent xenobiotic metabolism phase II and phase III enzymes differently in human colorectal cancer and untransformed epithelial colon cells. Nutr. Cancer 68 (8), 1338–1348. 10.1080/01635581.2016.1224369 [DOI] [PubMed] [Google Scholar]
- Mahran Y. F. (2020). New insights into the protection of growth hormone in cisplatin-induced nephrotoxicity: the impact of IGF-1 on the keap1-nrf2/HO-1 signaling. Life Sci. 253, 117581. 10.1016/j.lfs.2020.117581 [DOI] [PubMed] [Google Scholar]
- Manigandan K., Manimaran D., Jayaraj R. L., Elangovan N., Dhivya V., Kaphle A. (2015). Taxifolin curbs NF-κB-mediated Wnt/β-catenin signaling via up-regulating Nrf2 pathway in experimental colon carcinogenesis. Biochimie 119, 103–112. 10.1016/j.biochi.2015.10.014 [DOI] [PubMed] [Google Scholar]
- Mann G. E., Rowlands D. J., Li F. Y., de Winter P., Siow R. C. (2007). Activation of endothelial nitric oxide synthase by dietary isoflavones: role of NO in Nrf2-mediated antioxidant gene expression. Cardiovasc. Res. 75 (2), 261–274. 10.1016/j.cardiores.2007.04.004 [DOI] [PubMed] [Google Scholar]
- McCann S. M., Haens G., Mastronardi C., Walczewska A., Karanth S., Rettori V., et al. (2003). The role of nitric oxide (NO) in control of LHRH release that mediates gonadotropin release and sexual behavior. Curr. Pharm. Des. 9 (5), 381–390. 10.2174/1381612033391766 [DOI] [PubMed] [Google Scholar]
- McCann S. M., Karanth S., Mastronardi C. A., Dees W. L., Childs G., Miller B., et al. (2001). Control of gonadotropin secretion by follicle-stimulating hormone-releasing factor, luteinizing hormone-releasing hormone, and leptin. Arch. Med. Res. 32 (6), 476–485. 10.1016/s0188-4409(01)00343-5 [DOI] [PubMed] [Google Scholar]
- Mehnati P., Baradaran B., Vahidian F., Nadiriazam S. (2020). Functional response difference between diabetic/normal cancerous patients to inflammatory cytokines and oxidative stresses after radiotherapy. Rep. Pract. Oncol. Radiother. 25 (5), 730–737. 10.1016/j.rpor.2020.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melov S., Doctrow S. R., Schneider J. A., Haberson J., Patel M., Coskun P. E., et al. (2001). Lifepan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutasecatalase mimetics. J. Neurosci. 21 (21), 8348–8353. 10.1523/jneurosci.21-21-08348.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegon S., Columbano A., Giordano S. (2016). The dual roles of NRF2 in cancer. Trends Mol. Med. 22 (7), 578–593. 10.1016/j.molmed.2016.05.002 [DOI] [PubMed] [Google Scholar]
- Mishra P., Paital B., Jena S., Swain S. S., Kumar S., Yadav M. K., et al. (2019). Possible activation of NRF2 by Vitamin E/Curcumin against altered thyroid hormone induced oxidative stress via NFĸB/AKT/mTOR/KEAP1 signalling in rat heart. Sci. Rep. 9, 7408. 10.1038/s41598-019-43320-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno C. S., Evans C. O., Zhan X., Okor M., Desiderio D. M., Oyesiku N. M. (2005). Novel molecular signaling and classification of human clinically nonfunctional pituitary adenomas identified by gene expression profiling and proteomic analyses. Cancer Res. 65, 10214–10222. 10.1158/0008-5472.CAN-05-0884 [DOI] [PubMed] [Google Scholar]
- Nachliely M., Trachtenberg A., Khalfin B., Nalbandyan K., Cohen-Lahav M., Yasuda K., et al. (2019). Dimethyl fumarate and vitamin D derivatives cooperatively enhance VDR and Nrf2 signaling in differentiating AML cells in vitro and inhibit leukemia progression in a xenograft mouse model. J. Steroid Biochem. Mol. Biol. 188, 8–16. 10.1016/j.jsbmb.2018.11.017 [DOI] [PubMed] [Google Scholar]
- Neumann C. A., Krause D. S., Carman C. V., Das S., Dubey D. P., Abraham J. L., et al. (2003). Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature 424 (6948), 561–565. 10.1038/nature01819 [DOI] [PubMed] [Google Scholar]
- Niture S. K., Jaiswal A. K. (2010). Hsp90 interaction with INrf2(Keap1) mediates stress-induced Nrf2 activation. J. Biol. Chem. 285 (47), 36865–36875. 10.1074/jbc.M110.175802 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Obrador E., Liu-Smith F., Dellinger R. W., Salvador R., Meyskens F. L., Estrela J. M. (2019). Oxidative stress and antioxidants in the pathophysiology of malignant melanoma. Biol. Chem. 400 (5), 589–612. 10.1515/hsz-2018-0327 [DOI] [PubMed] [Google Scholar]
- Osburn W. O., Wakabayashi N., Misra V., Nilles T., Biswal S., Trush M. A., et al. (2006). Nrf2 regulates an adaptive response protecting against oxidative damage following diquat-mediated formation of superoxide anion. Arch. Biochem. Biophys. 454 (1), 7–15. 10.1016/j.abb.2006.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorelli D., Fabricio A. S. C., Giovanis P., D'Ippolito S., Fiduccia P., Soldà C., et al. (2018). Phytosome complex of curcumin as complementary therapy of advanced pancreatic cancer improves safety and efficacy of gemcitabine: results of a prospective phase II trial. Pharmacol. Res. 132, 72–79. 10.1016/j.phrs.2018.03.013 [DOI] [PubMed] [Google Scholar]
- Pawlikowshi M., Winczyk K., Jaranowska M. (2003). Immunohistochemical demonstration of nitric oxide synthase (NOS) in the normal rat pituitary gland, estrogen-induced rat pituitary tumor and human pituitary adenomas. Folia Histochem. Cytobiol 41 (2), 87–90. [PubMed] [Google Scholar]
- Pi J., Zhang Q., Woods C. G., Wong V., Collins S., Andersen M. E. (2008). Activation of Nrf2-mediated oxidative stress response in macrophages by hypochlorous acid. Toxicol. Appl. Pharmacol. 226 (3), 236–243. 10.1016/j.taap.2007.09.016 [DOI] [PubMed] [Google Scholar]
- Pinilla L., González L. C., Tena-Sempere M., Bellido C., Aguilar E. (2001). Effects of systemic blockade of nitric oxide synthases on pulsatile LH, prolactin, and GH secretion in adult male rats. Horm. Res. 55 (5), 229–235. 10.1159/000050001 [DOI] [PubMed] [Google Scholar]
- Pinilla L., Tena-Sempere M., Aguilar E. (1999). Nitric oxide stimulates growth hormone secretion in vitro through a calcium- and cyclic guanosine monophosphate-independent mechanism. Horm. Res. 51 (5), 242–247. 10.1159/000023378 [DOI] [PubMed] [Google Scholar]
- Pizza V., Agresta A., D’Acunto C. W., Festa M., Capasso A. (2011). Neuroinflammation and ageing: current theories and an overview of the data. Rev. Recent Clin. Trials 6 (3), 189–203. 10.2174/157488711796575577 [DOI] [PubMed] [Google Scholar]
- Prasad S., Gupta S. C., Pandey M. K., Tyagi A. K., Deb L. (2016). Oxidative stress and cancer: advances and challenges. Oxid Med. Cell Longev. 2016, 5010423. 10.1155/2016/5010423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevatto J. P., Torres R. C., Diaz B. L., Silva P. M. R. E., Martins M. A., Carvalho V. F. (2017). Antioxidant treatment induces hyperactivation of the HPA Axis by upregulating ACTH receptor in the adrenal and downregulating glucocorticoid receptors in the pituitary. Oxid Med. Cell Longev. 2017, 4156361. 10.1155/2017/4156361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S., Golubnitschaja O., Zhan X. (2019). Chronic inflammation: key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles. EPMA J. 10, 365–381. 10.1007/s13167-019-00194-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S., Yang Y., Li N., Cheng T., Wang X., Liu J., et al. (2018). Prolactin variants in human pituitaries and pituitary adenomas identified with two-dimensional gel electrophoresis and mass spectrometry. Front. Endocrinol. 9, 468. 10.3389/fendo.2018.00468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J. J., Cheng X. D., Zhang J., Zhang W. D. (2019). Dual roles and therapeutic potential of Keap1-Nrf2 pathway in pancreatic cancer: a systematic review. Cell Commun. Signal 17 (1), 121. 10.1186/s12964-019-0435-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimondo G., Puglisi S., Pia A., Terzolo M. (2019). Autonomous hypercortisolism: definition and clinical implications. Minerva. Endocrinol. 44, 33–42. 10.23736/s0391-1977.18.02884-5 [DOI] [PubMed] [Google Scholar]
- Reuter S., Gupta S. C., Chaturvedi M. M., Aggarwal B. B. (2010). Oxidative stress, inflammation, and cancer: how are they linked? Free Radic. Biol. Med. 49 (11), 1603–1616. 10.1016/j.freeradbiomed.2010.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel W. (2002). Role of nitric oxide in the control of the hypothalamic-pituitary-adrenocortical axis. Z. Rheumatol. 59 (Suppl. 2), 42. 10.1007/s003930070016 [DOI] [PubMed] [Google Scholar]
- Roy Chowdhury S., Sengupta S., Biswas S., Sinha T. K., Sen R., Basak R. K., et al. (2014). Bacterial fucose-rich polysaccharide stabilizes MAPK-mediated Nrf2/Keap1 signaling by directly scavenging reactive oxygen species during hydrogen peroxide-induced apoptosis of human lung fibroblast cells. PLoS One 9 (11), e113663. 10.1371/journal.pone.0113663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo I. G., Kwak M. K. (2018). Regulatory crosstalk between the oxidative stress-related transcription factor Nfe2l2/Nrf2 and mitochondria. Toxicol. Appl. Pharmacol. 359, 24–33. 10.1016/j.taap.2018.09.014 [DOI] [PubMed] [Google Scholar]
- Saad El-Din S., Rashed L., Medhat E., Emad Aboulhoda B., Desoky Badawy A., Mohammed ShamsEldeen A., et al. (2020). Active form of vitamin D analogue mitigates neurodegenerative changes in Alzheimer's disease in rats by targeting Keap1/Nrf2 and MAPK-38p/ERK signaling pathways. Steroids 156, 108586. 10.1016/j.steroids.2020.108586 [DOI] [PubMed] [Google Scholar]
- Sabatino M. E., Grondona E., Sosa L. D. V., Mongi Bragato B., Carreño L., Juarez V., et al. (2018). Oxidative stress and mitochondrial adaptive shift during pituitary tumoral growth. Free Radic. Biol. Med. 120, 41–55. 10.1016/j.freeradbiomed.2018.03.019 [DOI] [PubMed] [Google Scholar]
- Sahin K., Orhan C., Akdemir F., Tuzcu M., Iben C., Sahin N. (2012). Resveratrol protects quail hepatocytes against heat stress: modulation of the Nrf2 transcription factor and heat shock proteins. J. Anim. Physiol. Anim. Nutr. 96 (1), 66–74. 10.1111/j.1439-0396.2010.01123.x [DOI] [PubMed] [Google Scholar]
- Sajadimajd S., Khazaei M. (2018). Oxidative stress and cancer: the role of Nrf2. Curr. Cancer Drug Targets 18 (6), 538–557. 10.2174/1568009617666171002144228 [DOI] [PubMed] [Google Scholar]
- Sánchez-Martín P., Sou Y. S., Kageyama S., Koike M., Waguri S., Komatsu M. (2020). NBR1-mediated p62-liquid droplets enhance the Keap1-Nrf2 system. EMBO Rep. 21 (3), e48902. 10.15252/embr.201948902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivils J. C., Ancrum T. M., Bain L. J. (2013). LOSS of Mrp1 alters detoxification enzyme expression in a tissue- and hormonal-status-specific manner. J. Appl. Toxicol. 33 (8), 766–773. 10.1002/jat.2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M. K., Lee J. H., Ryoo I. G., Lee S. H., Ku S. K., Kwak M. K. (2019). Bardoxolone ameliorates TGF-β1-associated renal fibrosis through Nrf2/Smad7 elevation. Free Radic. Biol. Med. 138, 33–42. 10.1016/j.freeradbiomed.2019.04.033 [DOI] [PubMed] [Google Scholar]
- Song N. Y., Kim D. H., Kim E. H., Na H. K., Kim N. J., Suh Y. G., et al. (2011). Multidrug resistance-associated protein 1 mediates 15-deoxy-Δ(12,14)-prostaglandin J2-induced expression of glutamate cysteine ligase expression via Nrf2 signaling in human breast cancer cells. Chem. Res. Toxicol. 24 (8), 1231–1241. 10.1021/tx200090n [DOI] [PubMed] [Google Scholar]
- Stalla G. K., Ciato D., Dimopoulou C. (2019). “The adrenal gland: central relay in health and disease - current challenges and perspectives 2018” - Cushing’s disease. Exp. Clin. Endocrinol. Diabetes 127, 147–155. 10.1055/a-0664-7632 [DOI] [PubMed] [Google Scholar]
- Sun T., Gao J., Han D., Shi H., Liu X. (2019). Fabrication and characterization of solid lipid nano-formulation of astraxanthin against DMBA-induced breast cancer via Nrf-2-Keap1 and NF-kB and mTOR/Maf-1/PTEN pathway. Drug Deliv. 26 (1), 975–988. 10.1080/10717544.2019.1667454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syu J. P., Chi J. T., Kung H. N. (2016). Nrf2 is the key to chemotherapy resistance in MCF7 breast cancer cells under hypoxia. Oncotarget 7 (12), 14659–14672. 10.18632/oncotarget.7406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi K., Yamamoto M. (2017). The KEAP1-NRF2 system in Cancer. Front. Oncol. 7, 85. 10.3389/fonc.2017.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B., Lu Z. N., Guo X. L. (2018). Regulation and role of nuclear factor-E2-related factor 2 (Nrf2) in multidrug resistance of hepatocellular carcinoma. Chem. Biol. Interact 280, 70–76. 10.1016/j.cbi.2017.12.014 [DOI] [PubMed] [Google Scholar]
- Tian H., Zhang D., Gao Z., Li H., Zhang B., Zhang Q., et al. (2014). MDA-7/IL-24 inhibits Nrf2-mediated antioxidant response through activation of p38 pathway and inhibition of ERK pathway involved in cancer cell apoptosis. Cancer Gene Ther. 21 (10), 416–426. 10.1038/cgt.2014.45 [DOI] [PubMed] [Google Scholar]
- Tian L., Lu Y., Yang T., Deng Z., Xu L., Yao W., et al. (2019). aPKCι promotes gallbladder cancer tumorigenesis and gemcitabine resistance by competing with Nrf2 for binding to Keap1. Redox Biol. 22, 101149. 10.1016/j.redox.2019.101149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To C., Ringelberg C. S., Royce D. B., Williams C. R., Risingsong R., Sporn M. B., et al. (2015). Dimethyl fumarate and the oleanane triterpenoids, CDDO-imidazolide and CDDO-methyl ester, both activate the Nrf2 pathway but have opposite effects in the A/J model of lung carcinogenesis. Carcinogenesis 36 (7), 769–781. 10.1093/carcin/bgv061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueta Y., Levy A., Powell M. P., Lightman S. L., Kinoshita Y., Yokota A., et al. (1998). Neuronal nitric oxide synthase gene expression in human pituitary tumours: a possible association with somatotroph adenomas and growth hormone-releasing hormone gene expression. Clin. Endocrinol. 49 (1), 29–38. 10.1046/j.1365-2265.1998.00399.x [DOI] [PubMed] [Google Scholar]
- Valko M., Rhodes C. J., Moncol J., Izakovic M., Mazur M. (2006). Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact 160 (1), 1–40. 10.1016/j.cbi.2005.12.009 [DOI] [PubMed] [Google Scholar]
- Vankelecom H., Matthys P., Denef C. (1997). Inducible nitric oxide synthase in the anterior pituitary gland: induction by interferon-r in a subpopulation of folliculostellate cells and in an unidentifiable population of nonhormone-secreting cells. J. Histochem. Cytochem. 45 (6), 847–857. 10.1177/002215549704500609 [DOI] [PubMed] [Google Scholar]
- Villeneuve N. F., Lau A., Zhang D. D. (2010). Regulation of the Nrf2–Keap1 antioxidant response by the ubiquitin proteasome system: an insight into cullin-ring ubiquitin ligases. Antioxid. Redox Signal. 13 (11), 1699–1712. 10.1089/ars.2010.3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K.-C., Liu Y.-C., El-Shazly M., Shih S.-P., Du Y.-C., Hsu Y.-M., et al. (2019). The antioxidant from ethanolic extract of Rosa cymosa fruits activates phosphatase and tensin homolog in vitro and in vivo: a new insight on its antileukemic effect. Int. J. Mol. Sci. 20 (8), 1935. 10.3390/ijms20081935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Shi G., Bian C., Nisar M. F., Guo Y., Wu Y., et al. (2018). UVA irradiation enhances brusatol-mediated inhibition of melanoma growth by downregulation of the Nrf2-mediated antioxidant response. Oxid Med. Cell Longev. 2018, 9742154. 10.1155/2018/9742154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Guo T., Peng F., Long Y., Mu Y., Yang H., et al. (2015). Proteomic and functional profiles of a follicle-stimulating hormone positive human nonfunctional pituitary adenoma. Electrophoresis 36, 1289–1304. 10.1002/elps.201500006 [DOI] [PubMed] [Google Scholar]
- Wang Y., Cheng T., Lu M., Mu Y., Li B., Li X., et al. (2019). TMT-based quantitative proteomics revealed follicle-stimulating hormone (FSH)-related molecular characterizations for potentially prognostic assessment and personalized treatment of FSH-positive non-functional pituitary adenomas. EPMA J. 10, 395–414. 10.1007/s13167-019-00187-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang J., Huang Z. H., Huang X. H., Zheng W. B., Yin X. F., et al. (2017). Isodeoxyelephantopin induces protective autophagy in lung cancer cells via Nrf2-p62-keap1 feedback loop. Cell Death Dis. 8 (6), e2876. 10.1038/cddis.2017.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Y., Yang Y. X., Zhao R., Pan S. T., Zhe H., He Z. X., et al. (2015). Bardoxolone methyl induces apoptosis and autophagy and inhibits epithelial-to-mesenchymal transition and stemness in esophageal squamous cancer cells. Drug Des. Devel Ther. 9, 993–1026. 10.2147/DDDT.S73493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfel N. A., Sainz A. G., Song J. H., Kraft A. S. (2016). PIM kinase inhibitors kill hypoxic tumor cells by reducing Nrf2 signaling and increasing reactive oxygen species. Mol. Cancer Ther. 15 (7), 1637–1647. 10.1158/1535-7163.MCT-15-1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Zhang H., Zhang J., Zhang H., Zeng Y., Fang S., et al. (2019). Increased oxidative stress, inflammation and fibrosis in perirenal adipose tissue of patients with cortisol-producing adenoma. Adipocyte 8 (1), 347–356. 10.1080/21623945.2019.1690834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zhang C., Zhang W., Shi R., Zhang Z. (2012). Extracellular superoxide dismutase, a potential extracellular biomarker candidate for prolactinoma. West Indian Med. J. 61 (7), 665–669. [PubMed] [Google Scholar]
- Yates M. S., Kensler T. W. (2007). Chemopreventive promise of targeting the Nrf2 pathway. Drug News Perspect. 20 (2), 109–117. 10.1358/dnp.2007.20.2.108343 [DOI] [PubMed] [Google Scholar]
- Yu Z., Dai Z. Y., Qin G. X., Li M. Y., Wu L. F. (2020). Alleviative effects of dietary microbial floc on copper-induced inflammation, oxidative stress, intestinal apoptosis and barrier dysfunction in Rhynchocypris lagowski Dybowski. Fish Shellfish Immunol. 106, 120–132. 10.1016/j.fsi.2020.07.070 [DOI] [PubMed] [Google Scholar]
- Zeng Y., Lian S., Li D., Lin X., Chen B., Wei H., et al. (2017). Anti-hepatocarcinoma effect of cordycepin against NDEA-induced hepatocellular carcinomas via the PI3K/Akt/mTOR and Nrf2/HO-1/NF-κB pathway in mice. Biomed. Pharmacother. 95, 1868–1875. 10.1016/j.biopha.2017.09.069 [DOI] [PubMed] [Google Scholar]
- Zhai X., Yuan S., Yang X., Zou P., Li L., Li G., et al. (2019). Chitosan oligosaccharides induce apoptosis in human renal carcinoma via reactive-oxygen-species-dependent endoplasmic reticulum stress. J. Agric. Food Chem. 67 (6), 1691–1701. 10.1021/acs.jafc.8b06941 [DOI] [PubMed] [Google Scholar]
- Zhan X., Desiderio D. M. (2003). A reference map of a human pituitary adenoma proteome. Proteomics 3, 699–713. 10.1002/pmic.200300408 [DOI] [PubMed] [Google Scholar]
- Zhan X., Desiderio D. M. (2006). Nitroproteins from a human pituitary adenoma tissue discovered with a nitrotyrosine affinity column and tandem mass spectrometry. Anal. Biochem. 354, 279–289. 10.1016/j.ab.2006.05.024 [DOI] [PubMed] [Google Scholar]
- Zhan X., Desiderio D. M. (2010a). Signaling pathway networks mined from human pituitary adenoma proteomics data. BMC Med. Genomics 3, 13. 10.1186/1755-8794-3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Desiderio D. M. (2010b). The use of variations in proteomes to predict, prevent, and personalize treatment for clinically nonfunctional pituitary adenomas. EPMA J. 1, 439–459. 10.1007/s13167-010-0028-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Desiderio D. M. (2004). The human pituitary nitroproteome: detection of nitrotyrosyl-proteins with two-dimensional Western blotting, and amino acid sequence determination with mass spectrometry. Biochem. Biophys. Res. Commun. 325, 1180–1186. 10.1016/j.bbrc.2004.10.169 [DOI] [PubMed] [Google Scholar]
- Zhan X., Wang X., Cheng T. (2016). Human pituitary adenoma proteomics: new progresses and perspectives. Front. Endocrinol. 7, 54. 10.3389/fendo.2016.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Wang X., Desiderio D. M. (2013). Pituitary adenoma nitroproteomics: current status and perspectives. Oxid Med. Cel Longev. 2013, 580710. 10.1155/2013/580710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Wang X., Long Y., Desiderio D. M. (2014a). Heterogeneity analysis of the proteomes in clinically nonfunctional pituitary adenomas. BMC Med. Genomics 7, 69. 10.1186/s12920-014-0069-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Desiderio D. M., Wang X., Zhan X., Guo T., Li M. (2014b). Identification of the proteomic variations of invasive relative to non-invasive non-functional pituitary adenomas. Electrophoresis 35, 2184–2194. 10.1002/elps.201300590 [DOI] [PubMed] [Google Scholar]
- Zhan X., Desiderio D. M. (2007). Linear ion-trap mass spectrometric characterization of human pituitary nitrotyrosine-containing proteins. Int. J. Mass. Spectrom. 259, 96–104. 10.1016/j.ijms.2006.06.009 [DOI] [Google Scholar]
- Zhang B., Ma Z., Tan B., Lin N. (2019). Targeting the cell signaling pathway Keap1-Nrf2 as a therapeutic strategy for adenocarcinomas of the lung. Expert Opin. Ther. Targets 23 (3), 241–250. 10.1080/14728222.2019.1559824 [DOI] [PubMed] [Google Scholar]
- Zhang J., Su L., Ye Q., Zhang S., Kung H., Jiang F., et al. (2017). Discovery of a novel Nrf2 inhibitor that induces apoptosis of human acute myeloid leukemia cells. Oncotarget 8 (5), 7625–7636. 10.18632/oncotarget.13825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Qiao R., He D., Zhao Z., Yang S., Zou H., et al. (2016). Indazolo[3,2-b]quinazolinones attack hepatocellular carcinoma Hep3B cells by inducing mitochondrial-dependent apoptosis and inhibition of Nrf2/ARE signaling pathway. Curr. Mol. Med. 16 (9), 820–828. 10.2174/1566524016666161128114444 [DOI] [PubMed] [Google Scholar]
- Zhao L., Liu Z., Jia H., Feng Z., Liu J., Li X. (2015). Lipoamide acts as an indirect antioxidant by simultaneously stimulating mitochondrial biogenesis and phase II antioxidant enzyme systems in ARPE-19 cells. PLoS One 10 (6), e0128502. 10.1371/journal.pone.0128502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. J., Zhao Z., Yang D. D., Cao L. L., Zhang L., Ji J., et al. (2017). Activation of ATP-sensitive potassium channel by iptakalim normalizes stress-induced HPA axis disorder and depressive behaviour by alleviating inflammation and oxidative stress in mouse hypothalamus. Brain Res. Bull. 130, 146–155. 10.1016/j.brainresbull.2017.01.026 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Giorgianni F., Desiderio D. M., Fang B., Beranova-Giorgianni S. (2005). Toward a global analysis of the human pituitary proteome by multiple gel-based technology. Anal. Chem. 77, 5324–5331. 10.1021/ac050354e [DOI] [PubMed] [Google Scholar]
- Zhou W., Song Y., Xu H., Zhou K., Zhang W., Chen J., et al. (2011). Nonfunctional pituitary adenomas, estrogen receptors and slug contribute to development of invasiveness. J. Clin. Endocrinol. Metab. 96, E1237–E1245. 10.1210/jc.2010-3040 [DOI] [PubMed] [Google Scholar]
- Zhu J., Wang H., Fan Y., Lin Y., Zhang L., Ji X., et al. (2014). Targeting the NF-E2-related factor 2 pathway: a novel strategy for glioblastoma. Oncol. Rep. 32 (2), 443–450. 10.3892/or.2014.3259 [DOI] [PubMed] [Google Scholar]