Figure EV4. Ndc80 phospho‐mutants modify kinetochore–microtubule attachment stability.
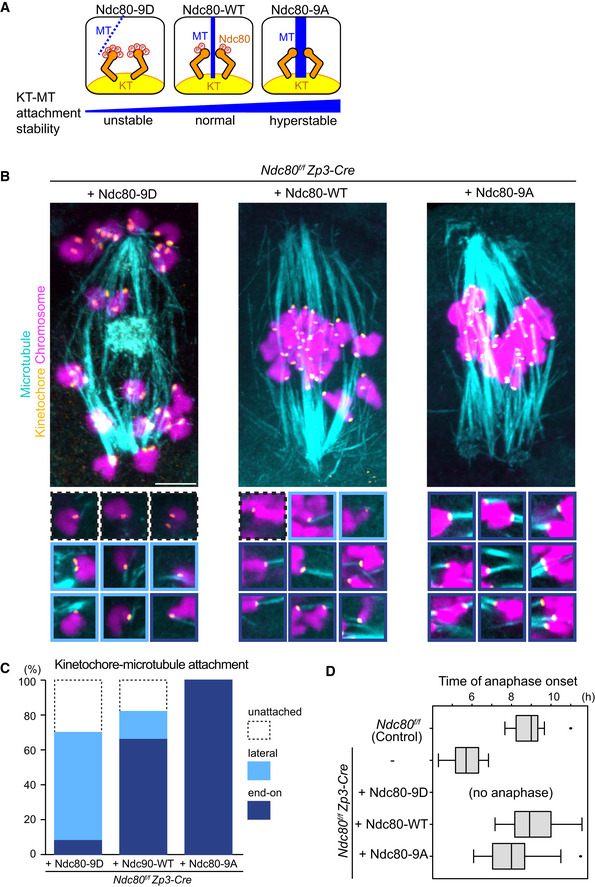
- Schematic representation of kinetochore–microtubule attachment. Phosphorylation levels of the Ndc80 complex at the kinetochore are a determinant of the stability of kinetochore (KT)‐microtubule (MT) attachments. High phosphorylation levels allow unstable attachments such as lateral attachments. Low phosphorylation levels promote stable attachments such as end‐on attachments. Phospho‐mimetic and phospho‐deficient forms of Ndc80 (S4, S5, T8, S15, S44, T49, S55, S62, and S68 were substituted to aspartic acid “Ndc80‐9D” or alanine “Ndc80‐9A”, respectively) can be used as tools to manipulate the stability of kinetochore–microtubule attachments.
- Ndc80 phospho‐mutants modify kinetochore–microtubule attachment stability. Ndc80f/f Zp3‐Cre oocytes were collected and microinjected with RNAs of wild‐type (Ndc80‐WT), phospho‐mimetic (Ndc80‐9D) or phospho‐deficient (Ndc80‐9A) forms of Ndc80. Cold‐stable microtubules were visualized by immunostaining oocytes 7 hours after NEBD for microtubules (α‐tubulin, cyan), kinetochores (ACA, orange), and chromosomes (Hoechst33342, magenta). Z‐projection images are shown. Magnified images of kinetochores are categorized: end‐on attachments (dark blue box), lateral attachments (light blue box), and unattached (dot‐lined box). Scale bar, 10 μm.
- Percentages of kinetochore–microtubule attachments in (B). All kinetochores were analyzed (n = 200 kinetochores of 5 oocytes from two independent experiments).
- Anaphase entry timing. Ndc80f/f (Ndc80‐intact) oocytes are used as a control. Ndc80f/f Zp3‐Cre (Ndc80‐deleted) oocytes expressing Ndc80‐WT, Ndc80‐9D, or Ndc80‐9A were monitored with the chromosome marker H2B‐mCherry (n = 15, 25, 25, 42, and 55 oocytes, respectively, from at least 3 independent experiments). Time after NEBD. Note that Ndc80f/f Zp3‐Cre oocytes expressing no Ndc80 construct exhibited an accelerated anaphase onset that is consistent with defects in the spindle checkpoint. Ndc80‐9D‐expressing oocytes did not undergo anaphase, consistent with spindle checkpoint activation by unstable kinetochore–microtubule attachments. Ndc80‐9A‐expressing oocytes tended to exhibit an earlier anaphase onset, in agreement with accelerated checkpoint satisfaction by stable kinetochore–microtubule attachments. Boxes show 25–75 percentiles and whiskers encompass data points within 1.5 times the interquartile range.
Source data are available online for this figure.
