Abstract
Introduction
Terminal complement amplification is hypothesized to be a key contributor to the clinical manifestations of severe coronavirus disease 2019 (COVID-19). Ravulizumab, a humanized monoclonal antibody that binds with high affinity to complement protein C5 and inhibits terminal complement activation, is being evaluated as a treatment for COVID-19-related severe pneumonia, acute lung injury, and acute respiratory distress syndrome in an ongoing phase 3 randomized controlled trial (ALXN1210-COV-305). To address the overactivation of terminal complement in severe COVID-19 compared to the diseases in which ravulizumab is currently approved, a modified dosing regimen was adopted. This analysis evaluates preliminary pharmacokinetic/pharmacodynamic data to confirm the modified dosing regimen.
Methods
Weight-based ravulizumab doses were administered on days 1, 5, 10, and 15. Serum levels of ravulizumab and free C5 were measured before and after administration of ravulizumab and any time on day 22. Free C5 levels < 0.5 μg/mL indicate complete C5 inhibition. The pharmacokinetic target was defined as ravulizumab concentrations at the end of the dosing interval > 175 μg/mL, the concentration above which C5 is completely inhibited.
Results
Twenty-two patients were included in this evaluation. At baseline, mean C5 concentration was 240 ± 67 μg/mL. In all patients and at all individual timepoints after the first dose was administered, ravulizumab concentrations remained > 175 μg/mL and free C5 concentrations remained < 0.5 μg/mL.
Conclusion
High levels of baseline C5 observed in patients with severe COVID-19 contribute to the growing body of evidence that suggests this disease is marked by amplification of terminal complement activation. Data from this preliminary pharmacokinetic/pharmacodynamic evaluation of 22 patients with severe COVID-19 show that the modified ravulizumab dosing regimen achieved immediate and complete terminal complement inhibition, which can be sustained for up to 22 days. These data support the continued use of this dosage regimen in the ongoing phase 3 study.
Trial Registration
ClinicalTrials.gov identifier, NCT04369469
Keywords: Antibodies, Complement C5/antagonists and inhibitors, Complement inactivating agents/therapeutic use, Coronavirus disease 2019, Humanized/therapeutic use, Monoclonal, Ravulizumab, Terminal complement pathway
Plain Language Summary
While many people have no or mild COVID-19 symptoms, a small number of people become very sick and require hospitalization in intensive care units. One part of their immune system, known as complement, overreacts and attacks the lungs and other organs. Researchers are looking for a way to keep the immune system from attacking the body instead of protecting it. Ravulizumab is a medication currently used to do this in other diseases. Ravulizumab is being studied to see if it can reduce the destructive and deadly effects of the coronavirus infection. In this evaluation, ravulizumab effectively reduced complement in patients with severe COVID-19.
Key Summary Points
| Why carry out this study? |
| There is an urgent clinical need for approved treatments that target immune system dysregulation and consequent amplification of the terminal complement pathway in patients with severe acute respiratory distress syndrome related to coronavirus disease 2019 (COVID-19). |
| Ravulizumab, a humanized monoclonal antibody that binds with high affinity to C5 and inhibits terminal complement activation, is being evaluated in an ongoing phase 3 randomized controlled trial (ALXN1210-COV-305) in patients with COVID-19 severe pneumonia, acute lung injury, or acute respiratory distress syndrome. |
| This evaluation assesses whether the modified dosing regimen selected to mitigate the amplification of terminal complement results in immediate and complete terminal complement inhibition. |
| What was learned from the study? |
| The high baseline serum C5 levels observed in patients with severe COVID-19 contributes to the growing body of evidence that suggests this disease is marked by amplification of terminal complement activation. |
| This evaluation of preliminary pharmacokinetic/pharmacodynamic data from 22 patients with severe COVID-19 shows that the modified ravulizumab dosing regimen achieved immediate and complete terminal complement inhibition for up to 22 days and supports the continued use of the dosage regimen in the ongoing phase 3 study. |
Digital Features
This article is published with digital features, including a summary slide and plain language summary, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14113445.
Introduction
Severe coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and is characterized by acute respiratory distress syndrome in approximately one-third of hospitalized patients [1]. Though the pathophysiology of the severe disease is still under investigation, it appears to be characterized by a dysregulation of the host immune response, by a proinflammatory, hypercoagulable state, and potentially by alterations in the complement system [2–5]. In particular, data collected from small groups of patients have shown that elements of the terminal complement pathway are amplified beyond what is expected in a typical immune response to viral infections [6–13]. Complement component C5a is an anaphylatoxin that recruits neutrophils, amplifies NETosis, and induces the prothrombotic state [2]. The membrane attack complex (C5b-9) directly promotes platelet adhesion and coagulation and can cause endothelial inflammation and microvascular injury [2]. These C5 activation products are elevated in patients with severe COVID-19 and increase with disease severity [2, 5–13].
While these data do not allow us to determine whether complement overactivation is a marker or a driver of disease progression, a small body of evidence may suggest a causal link. Mouse models of severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) have shown that compared to wild-type controls, complement-deficient mice infected with SARS-CoV or MERS-CoV develop less respiratory dysfunction, less lung tissue damage, and lower levels of tissue and systemic inflammation [14, 15]. In addition, case reports and one small, proof-of-concept, compassionate-use study suggest that inhibition of terminal complement at C5, the precursor to C5a and C5b-9, may interrupt tissue-damaging feedback loops and improve clinical outcomes and prognosis [6, 16–18]. Together these data suggest that C5 inhibition may represent a potential therapeutic target for severe COVID-19.
Ravulizumab (Ultomiris™; Alexion Pharmaceuticals, Inc., Boston, MA, USA) is a humanized monoclonal antibody that binds with high affinity to C5 [19] and inhibits its cleavage into C5a and C5b. Ravulizumab is efficacious and safe for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS), two diseases in which complement levels are moderately elevated [20–22]. In these immunomodulatory diseases, ravulizumab inhibits the terminal complement pathway; and as a result, pathological sequelae, which develop from uncontrolled terminal complement activation, such as cell death and tissue damage, are obviated.
To determine whether treatment with ravulizumab can improve outcomes in patients with COVID-19 severe pneumonia, acute lung injury, or acute respiratory distress syndrome, a phase 3, open-label randomized controlled trial (ALXN1210-COV-305; NCT04369469) is underway [23]. As terminal complement may be more highly amplified in patients with severe COVID-19 than in patients with PNH or aHUS, [6, 16, 20–22], a modified dosing regimen was adopted. This preliminary pharmacokinetic (PK)/pharmacodynamic (PD) evaluation was undertaken to document levels of C5 in patients with severe COVID-19 and to determine if the modified dosing regimen provides immediate and complete C5 inhibition in these patients [23]. The protocol-specified outcomes will be the subject of a separate analysis.
Methods
A detailed protocol description of ALXN1210-COV-305 has been published [23]. In brief, ALXN1210-COV-305 is an ongoing phase 3, open-label randomized controlled trial. Patients with a confirmed diagnosis of SARS-CoV-2 infection requiring hospitalization and mechanical ventilation (invasive or non-invasive) due to COVID-19 severe pneumonia, acute lung injury, or acute respiratory distress syndrome were enrolled. Patients were excluded if they were not expected to survive for more than 24 h; if they had been on invasive mechanical ventilation with intubation for more than 48 h prior to screening; if they had severe pre-existing cardiac disease or an unresolved Neisseria meningitidis infection; or if they were under current treatment with a complement inhibitor, under intravenous immunoglobulin treatment within 4 weeks prior to randomization, or under current or prior (previous 30 days) treatment with an investigational therapy. Investigational therapies and antiviral therapies (such as remdesivir) were allowed if they were received as part of best supportive care (BSC) through an expanded access protocol or emergency approval for the treatment of COVID-19.
Patients were randomized to BSC or ravulizumab + BSC [20, 23]. The modified dosing schedule was designed to accommodate the expected higher baseline levels of C5, to sustain serum levels of ravulizumab, and to maintain C5 inhibition until day 29 [23]. These doses were based on dose simulations performed using a ravulizumab population PK model with an additional modification to accommodate the suspected faster anti-C5 antibody clearance in diseases that severely activate the terminal complement pathway. Weight-based ravulizumab doses were administered on days 1, 5, 10, and 15. On day 1, patients were administered the standard dose of ravulizumab: 2400 mg, 2700 mg, or 3000 mg if they weighed ≥ 40 to < 60 kg, 60 to < 100 kg, or ≥ 100 kg, respectively. Additional doses that are not approved for PNH and aHUS [20] were administered on days 5 and 10; patients were administered 600 mg if they weighed < 60 kg and 900 mg if they weighed ≥ 60 kg. On day 15, all patients were administered ravulizumab 900 mg, a modified dose that was selected to sustain serum levels of ravulizumab and maintain C5 inhibition until day 29 [23].
This PK/PD evaluation included patients randomized to the ravulizumab + BSC group. The cutoff for patient inclusion was predefined as the point at which the first 10 patients in the ravulizumab + BSC group had received the day 15 dose. Patients who had received at least one dose of ravulizumab at the time of cutoff were included in the analysis. Data from patients who withdrew from treatment were included until the time of withdrawal. Data up to the day 29 timepoint were included.
Blood samples were collected at baseline, before and after administration of each ravulizumab dose on days 1, 5, 10, and 15, and any time on day 22 and day 29. Assays used to measure serum ravulizumab levels and serum free C5 were performed as previously described [21]. Free C5 levels < 0.5 μg/mL indicate complete C5 inhibition (target PD threshold) [20]. A ravulizumab concentration at the end of the dosing interval (Ctrough) > 175 μg/mL indicates a near maximal PD effect (target PK threshold) [24].
Statistical analyses were descriptive. No sample size calculations were performed for this preliminary PK/PD evaluation. Data for missing timepoints were not imputed.
Compliance with Ethics Guidelines
Study ALXN1210-COV-305 is ongoing and is being conducted in accordance with the protocol; all applicable government regulations; the consensus ethical principles derived from international guidelines including the Declaration of Helsinki 1964, and its later amendments; the Council for International Organizations of Medical Sciences International Ethical Guidelines; and applicable International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice Guidelines. Ethics Committee approval was obtained from the Western Institutional Review Board ([IRB] central IRB tracking number, 20201059; work order number, 1-1297217-1; date of approval, 23 April 2020) and from available local IRBs (local IRB not available for two sites). Patients or their legal representative provided written informed consent. If the patient and legal representative were unable to provide informed consent and if local regulations allowed it, exceptions could be granted per the judgement of the investigator or designee. Written certification from the investigator and a physician who was not involved with the research was necessary and needed to be submitted to the IRB (local and central)/independent ethics committee within 5 working days of administration of the initial dose.
Results
At cutoff, 22 patients had been randomized to ravulizumab + BSC and had received at least one dose of ravulizumab. Fifty-five percent of patients were male (Table 1). At baseline, median age was 66 years (range 39–77 years). Median weight was 87.2 kg (range 63.5–160.0 kg); eight patients weighed ≥ 100 kg. The most common comorbidities were diabetes mellitus (50% of patients), hypertension (46%), hyperlipidemia (36%), and obesity (27%).
Table 1.
Demographics and baseline clinical characteristics
| Characteristic | Ravulizumab + BSC N = 22 |
|---|---|
| Male, n (%) | 12 (54.5) |
| Age in years | |
| Mean ± SD | 62 ± 11 |
| Median (min, max) | 66 (39, 77) |
| Age by category, n (%) | |
| < 50 years | 3 (13.6) |
| 50 to < 70 years | 12 (54.5) |
| ≥ 70 years | 7 (31.8) |
| Weight in kg | |
| Mean ± SD | 94.8 ± 22.7 |
| Median (min, max) | 87.2 (63.5, 160.0) |
| Weight by category, n (%) | |
| ≥ 40 to < 60 kg | 0 |
| ≥ 60 to < 100 kg | 14 (63.6) |
| ≥ 100 kg | 8 (36.4) |
| Country, n (%) | |
| GBR | 4 (18.2) |
| USA | 18 (81.8) |
| Race, n (%) | |
| White | 10 (45.5) |
| Black or African American | 6 (27.3) |
| Asian | 2 (9.1) |
| Other | 1 (4.5) |
| Unknown | 3 (13.6) |
| Medical conditionsa, n (%) | |
| Diabetes mellitus | 11 (50.0) |
| Hypertension | 10 (45.5) |
| Hyperlipidemia | 8 (36.4) |
| Obesityb | 6 (27.3) |
| Depression | 4 (18.2) |
| Drug hypersensitivity | 4 (18.2) |
| Gastroesophageal reflux disease | 4 (18.2) |
| Asthma | 3 (13.6) |
| Chronic kidney disease | 3 (13.6) |
| Hyperglycemia | 3 (13.6) |
| Hypokalemia | 3 (13.6) |
| Myocardial infarction | 3 (13.6) |
| Sleep apnea syndrome | 3 (13.6) |
BSC best supportive care, GBR Great Britain, min minimum, max maximum, SD standard deviation, USA United States of America
aCurrent or past non-acute comorbidities present in > 10% of patients
bPer physician assessment as many patients were proned and height was not measured
At baseline, the mean (± standard deviation) C5 concentration was 240 ± 67 µg/mL (range 106–343 μg/mL). Three patients had C5 levels < 150 μg/mL. All other patients (86%) had C5 levels > 190 μg/mL.
At cutoff, the number of patients who had received ravulizumab at the day 1, 5, 10, and 15 timepoints was 22, 16, 12, and 10, respectively. Three patients reached the day 22 timepoint and one patient reached the day 29 timepoint. The latter patient was excluded from the day 15, day 22, and day 29 PK/PD analysis after receiving multiple packed red blood cell transfusions starting on day 12. Thus, only two patients were included in the day 22 analysis and no patients were evaluable at day 29.
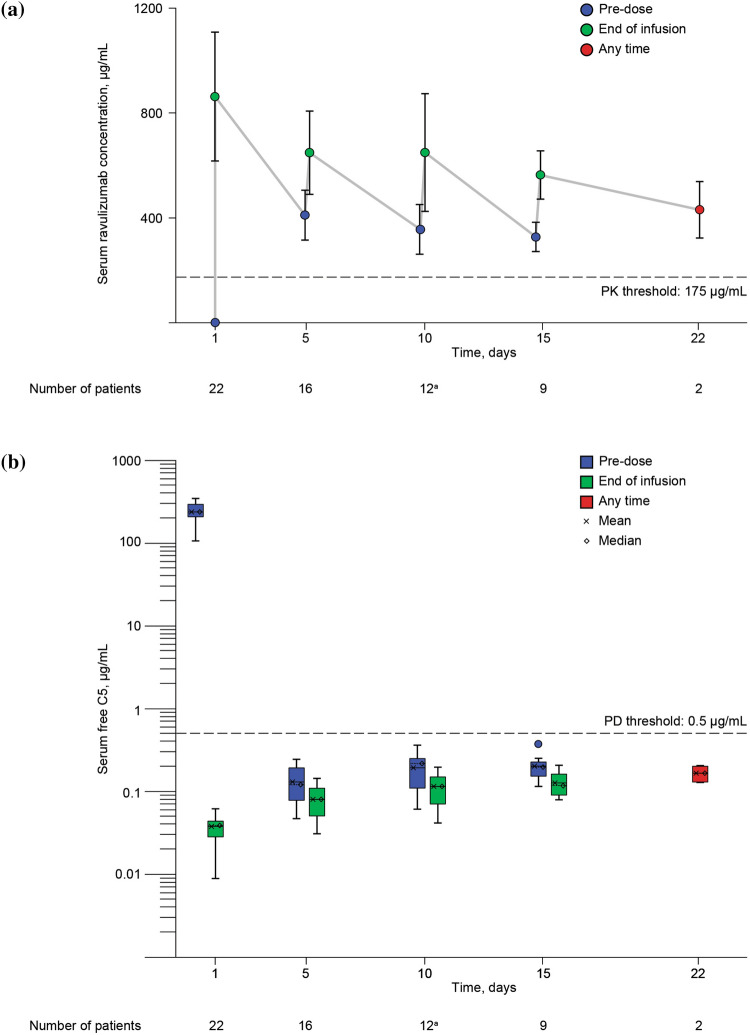
Administration of the first dose of ravulizumab increased serum ravulizumab levels above the target PK threshold of 175 μg/mL in all patients (Fig. 1a). Serum ravulizumab concentrations remained above 175 μg/mL in all patients and at all measured timepoints up to day 22. For all patients, serum free C5 concentrations dropped below 0.5 μg/mL (the target threshold for complete terminal complement inhibition) after administration of the first dose of ravulizumab (Fig. 1b). All individual serum free C5 levels measured until day 22 remained below 0.5 μg/mL.
Fig. 1.
Ravulizumab and free C5 concentrations over time. a Mean (± standard deviation) serum ravulizumab concentrations; b serum free C5 concentrations. Serum ravulizumab and free C5 concentrations were measured before and after ravulizumab was administered on study days 1, 5, 10, and 15, and any time on day 22. Complete terminal complement inhibition (PD threshold) was defined as serum free C5 levels < 0.5 μg/mL [20]. The PK threshold for maintaining complete terminal complement inhibition was defined as a serum ravulizumab concentration > 175 μg/mL [24]. an = 13 pre-dose; one patient withdrew after the day 10 pre-dose blood draw but prior to receiving the day 10 ravulizumab dose. In b, the top and the bottom of the box correspond to the 75th and 25th percentiles, respectively. The whiskers represent 1.5 times the interquartile range (75th percentile–25th percentile). PK pharmacokinetic, PD pharmacodynamic
Discussion
As terminal complement pathway amplification is associated with lung inflammation, lung damage, and respiratory distress in patients with severe COVID-19 [6–10, 16], inhibition of terminal complement at the level of C5 may reduce amplification of the inflammatory and hypercoagulation pathways without inhibiting upstream complement pathways, which preserve the innate immune response. Herein, we evaluated whether the modified ravulizumab dosing regimen selected for this study population resulted in terminal complement inhibition in the first 22 patients enrolled in the ravulizumab + BSC treatment group.
In these severe COVID-19 patients, baseline levels of C5 ranged from 106 to 343 μg/mL. These data suggest that compared to healthy individuals whose C5 levels are typically below 100 μg/mL [25], terminal complement levels were elevated in all 22 patients. Three patients had baseline levels of C5 that were similar to those reported in PNH and aHUS, which typically range from 100 to 150 μg/mL [21, 22], whereas 19 patients had levels that were quantitatively higher than those observed in PNH and aHUS (> 150 μg/mL). Variations in baseline terminal complement levels have also been reported in a study of 103 patients hospitalized with COVID-19, in which circulating sC5b-9 levels were significantly elevated in 64% of patients compared to healthy controls [6].
The dosing regimen implemented in this study resulted in ravulizumab concentrations remaining above the threshold of 175 μg/mL, which indicates a near maximal PD effect (complete C5 inhibition), at all measured timepoints including in the two patients at day 22. These data demonstrate that the modified dosing regimen achieved complete terminal complement inhibition with the first dose of ravulizumab and that complete terminal complement inhibition was likely to be sustained for at least 22 days with doses on days 1, 5, 10, and 15. Overall, these PK/PD data support the continued use of this modified dosing regimen in this setting. Analysis of the total study population data will determine the generalizability of these PK/PD results and whether inhibition of terminal complement results in improved clinical outcomes.
Though this evaluation included only 22 patients, baseline characteristics appear to be representative of patients who develop severe COVID-19 with respiratory distress and a need for mechanical ventilation. Patients with severe COVID-19 tend to be elderly, heavier, and have a history of hypertension, cardiovascular disease, or diabetes mellitus [26]. In this analysis, > 30% of patients were over the age of 70 years; and all 22 patients weighed at least 60 kg. A large percentage of patients reported diabetes mellitus, hypertension, hyperlipidemia, or obesity.
Kidney function, liver function, and age have not been shown to affect the PK/PD of ravulizumab in PNH and aHUS [20]. On the basis of the present analysis, which included elderly patients with comorbidities, it is not anticipated that further dose adjustments will be required for patients with severe COVID-19. Although the modified dosing regimen appears to accommodate the augmented complement activation successfully, there may be clinical circumstances, related to COVID-19, which require additional dose adjustments, such as blood transfusions.
Limitations
This PK/PD evaluation does not include clinical outcomes and therefore no inferences about the impact of ravulizumab on the course of disease can be made. The baseline C5 data reported herein suggest an association between severe COVID-19 and terminal complement upregulation, but do not inform whether C5 is a marker of disease severity or a contributor to the pathobiology. In addition, as there is a paucity of data describing C5 levels in viral infections, interpreting baseline C5 levels in the context of infectious disease is difficult. A control group was not included in this analysis; and therefore, inhibition of C5 levels can only be presumed to reflect ravulizumab inhibition of terminal complement. Descriptions of the change in free C5 levels in patients on BSC would be needed to support this conclusion. In this cohort, no patients weighed < 60 kg. Though additional data are needed, drug exposures are expected to be no lower in patients < 60 kg than those achieved in this evaluation. A full PK/PD characterization using model-based techniques and an outcome analysis of the whole cohort of study ALXN1210-COV-305 are planned.
Conclusions
These results contribute to the growing body of evidence that suggests that severe COVID-19 is a disease characterized by significant terminal complement amplification [6–10]. In this preliminary PK/PD evaluation of 22 patients with severe COVID-19 enrolled in the ALXN1210-COV-305 phase 3 study, a modified ravulizumab dosing regimen resulted in immediate and complete terminal complement inhibition that could be sustained for up to 22 days. Data from this analysis support the continued use of this dosage regimen in the ongoing phase 3 study.
Acknowledgements
The authors would like to thank the patients for their participation in this clinical study and investigators who enrolled patients included in this analysis: Dr C. Broome (Georgetown University, Washington, DC, USA), Dr G. Frendl (Brigham and Women's Hospital, Boston, MA, USA), Dr M. Khoshnevis (Houston Methodist Hospital, Houston, TX, USA), Dr A Kulasekararaj (King's College Hospital, London, England), Dr H. Kulkarni (Washington University School of Medicine, St. Louis, MO, USA), Dr S. Pittock and Dr B. Pickering (Mayo Clinic, Rochester, NY, USA), Dr S. Savic (St James's University Hospital, Leeds, England), Dr J. Siegel (Mayo Clinic, Jacksonville, FL USA).
Funding
This study was funded by Alexion Pharmaceuticals, Inc. (Boston, MA). Alexion Pharmaceuticals, Inc. was responsible for designing the study and running the PK/PD analysis. All authors are employees of Alexion Pharmaceuticals, Inc. The journal’s Rapid Service Fee was paid by Alexion Pharmaceuticals, Inc.
Medical Writing, Editorial, and Other Assistance
Medical writing and editorial support were provided by Hélène Dassule, PhD (consultant for Alexion Pharmaceuticals, Inc.) with funding from Alexion Pharmaceuticals, Inc. Editorial review for scientific accuracy was provided by Kenneth Pomerantz, PhD (Alexion Pharmaceuticals, Inc). We would like to thank Scott Rottinghaus, MD (Alexion Pharmaceuticals, Inc) for his assistance with the development of the protocol and critical review of the manuscript; and Amy Pace, ScD (Alexion Pharmaceuticals, Inc) for her critical review of the manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Alanna McEneny-King, Shamsah Kazani and Stephan Ortiz are employees and stockholders of Alexion Pharmaceuticals, Inc. Jonathan Monteleone is an employee of Alexion Pharmaceuticals, Inc and has a patent (63/065,107) pending.
Compliance with Ethics Guidelines
Study ALXN1210-COV-305 is ongoing and is being conducted in accordance with the protocol; all applicable government regulations; the consensus ethical principles derived from international guidelines including the Declaration of Helsinki 1964, and its later amendments; the Council for International Organizations of Medical Sciences International Ethical Guidelines; and applicable International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice Guidelines. Ethics Committee approval was obtained from the Western IRB (central IRB tracking number, 20201059; work order number, 1-1297217-1; date of approval, 23 April 2020) and from available local IRBs (local IRB not available for two sites). Patients or their legal representative provided written informed consent. If the patient and legal representative were unable to provide informed consent and if local regulations allowed it, exceptions could be granted per the judgement of the investigator or designee. Written certification from the investigator and a physician who was not involved with the research was necessary and needed to be submitted to the IRB (local and central)/independent ethics committee within 5 working days of administration of the initial dose.
Data Availability
All the results pertaining to this analysis are contained in this paper. Once the primary manuscript describing protocol-specified endpoints has been published, Alexion will consider requests for disclosure of clinical study participant-level data provided that participant privacy is assured through methods like data de-identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at https://alexion.com/our-research/research-and-development. Link to Data Request Form (https://www.alexion.com/contact-alexion/medical-information).
References
- 1.Tzotzos SJ, Fischer B, Fischer H, Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care. 2020;24(1):516. doi: 10.1186/s13054-020-03240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Java A, Apicelli AJ, Liszewski MK, et al. The complement system in COVID-19: friend and foe? JCI Insight. 2020;5(15):e140711. doi: 10.1172/jci.insight.140711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992 e3–1000 e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tiwari R, Mishra AR, Mikaeloff F, et al. In silico and in vitro studies reveal complement system drives coagulation cascade in SARS-CoV-2 pathogenesis. Comput Struct Biotechnol J. 2020;18:3734–3744. doi: 10.1016/j.csbj.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peffault de Latour R, Bergeron A, Lengline E, et al. Complement C5 inhibition in patients with COVID-19—a promising target? Haematologica. 2020;105(12):2847–2850. doi: 10.3324/haematol.2020.260117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holter JC, Pischke SE, de Boer E, et al. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc Natl Acad Sci USA. 2020;117(40):25018–25025. doi: 10.1073/pnas.2010540117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carvelli J, Demaria O, Vely F, et al. Association of COVID-19 inflammation with activation of the C5a–C5aR1 axis. Nature. 2020;588(7836):146–150. doi: 10.1038/s41586-020-2600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cugno M, Meroni PL, Gualtierotti R, et al. Complement activation and endothelial perturbation parallel COVID-19 severity and activity. J Autoimmun. 2021;116:102560. doi: 10.1016/j.jaut.2020.102560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Alessandro A, Thomas T, Dzieciatkowska M, et al. Serum proteomics in COVID-19 patients: altered coagulation and complement status as a function of IL-6 level. J Proteome Res. 2020;19(11):4417–4427. doi: 10.1021/acs.jproteome.0c00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramlall V, Thangaraj PM, Meydan C, et al. Immune complement and coagulation dysfunction in adverse outcomes of SARS-CoV-2 infection. Nat Med. 2020;26(10):1609–1615. doi: 10.1038/s41591-020-1021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diorio C, McNerney KO, Lambert M, et al. Evidence of thrombotic microangiopathy in children with SARS-CoV-2 across the spectrum of clinical presentations. Blood Adv. 2020;4(23):6051–6063. doi: 10.1182/bloodadvances.2020003471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gralinski LE, Sheahan TP, Morrison TE, et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio. 2018;9(5):e01753-18. doi: 10.1128/mBio.01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Y, Zhao G, Song N, et al. Blockade of the C5a–C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV. Emerg Microbes Infect. 2018;7(1):77. doi: 10.1038/s41426-018-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Annane D, Heming N, Grimaldi-Bensouda L, et al. Eculizumab as an emergency treatment for adult patients with severe COVID-19 in the intensive care unit: a proof-of-concept study. EClinicalMedicine. 2020;28:100590. doi: 10.1016/j.eclinm.2020.100590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diurno F, Numis FG, Porta G, et al. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci. 2020;24(7):4040–4047. doi: 10.26355/eurrev_202004_20875. [DOI] [PubMed] [Google Scholar]
- 18.Laurence J, Mulvey JJ, Seshadri M, et al. Anti-complement C5 therapy with eculizumab in three cases of critical COVID-19. Clin Immunol. 2020;219:108555. doi: 10.1016/j.clim.2020.108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheridan D, Yu ZX, Zhang Y, et al. Design and preclinical characterization of ALXN1210: a novel anti-C5 antibody with extended duration of action. PLoS One. 2018;13(4):e0195909. doi: 10.1371/journal.pone.0195909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.United States Food and Drug Administration . Ultomiris® (ravulizumab-cwvz) prescribing information. Boston: Alexion Pharmaceuticals; 2019. [Google Scholar]
- 21.Peffault de Latour R, Brodsky RA, Ortiz S, et al. Pharmacokinetic and pharmacodynamic effects of ravulizumab and eculizumab on complement component 5 in adults with paroxysmal nocturnal haemoglobinuria: results of two phase 3 randomised, multicentre studies. Br J Haematol. 2020;191(3):476–485. doi: 10.1111/bjh.16711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rondeau E, Scully M, Ariceta G, et al. The long-acting C5 inhibitor, ravulizumab, is effective and safe in adult patients with atypical hemolytic uremic syndrome naive to complement inhibitor treatment. Kidney Int. 2020;97(6):1287–1296. doi: 10.1016/j.kint.2020.01.035. [DOI] [PubMed] [Google Scholar]
- 23.Smith K, Pace A, Ortiz S, Kazani S, Rottinghaus S. A phase 3 open-label, randomized, controlled study to evaluate the efficacy and safety of intravenously administered ravulizumab compared with best supportive care in patients with COVID-19 severe pneumonia, acute lung injury, or acute respiratory distress syndrome: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):639. doi: 10.1186/s13063-020-04548-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexion Pharmaceuticals Inc. NDA/BLA multi-disciplinary review and evaluation BLA 761108. Ultomiris (ravulizumab) 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/761108Orig1s000MultidisciplineR.pdf. Accessed 30 Oct 2020.
- 25.Gaya da Costa M, Poppelaars F, van Kooten C, et al. Age and sex-associated changes of complement activity and complement levels in a healthy caucasian population. Front Immunol. 2018;9:2664. doi: 10.3389/fimmu.2018.02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karagiannidis C, Mostert C, Hentschker C, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8(9):853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the results pertaining to this analysis are contained in this paper. Once the primary manuscript describing protocol-specified endpoints has been published, Alexion will consider requests for disclosure of clinical study participant-level data provided that participant privacy is assured through methods like data de-identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at https://alexion.com/our-research/research-and-development. Link to Data Request Form (https://www.alexion.com/contact-alexion/medical-information).