Figure 3. Ubiquitin‐dependent regulation of transcriptional machinery.
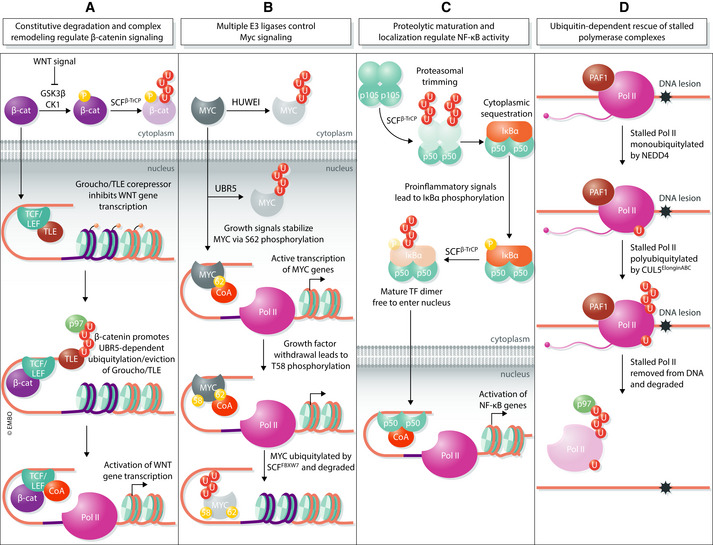
(A) In the absence of WNT signal, the β‐catenin transcription factor (β‐cat) is constitutively degraded by a destruction complex consisting of kinases GSK3β and CK1 as well as the ubiquitin ligase SCFβTrCP. The presence of WNT ligand stabilizes β‐catenin by inhibiting its phosphorylation, thus allowing it to translocate into the nucleus. At TCF/LEF transcriptional complexes, β‐catenin facilitates the exchange of co‐repressors for co‐activators (CoA) through UBR5‐mediated ubiquitylation and p97/VCP‐dependent extraction of the repressive Groucho/TLE subunit. (B) MYC levels are kept low in the cytoplasm and nucleus by the E3 ligases HUWE1 and UBR5, respectively. Upstream growth signals stabilize and activate MYC through phosphorylation of the S62 residue. This primes MYC for subsequent phosphorylation at nearby T58, which occurs upon growth factor withdrawal. T58‐phosphorylated MYC is recognized by the SCFFBXW7 E3 ligase and degraded to prevent subsequent re‐initiation of transcription. (C) The proinflammatory transcription factor NF‐κB is synthesized as a 105‐kDa precursor (p105) that is ubiquitylated by the SCFβTrCP E3 ligase and partially processed by the proteasome to yield a mature 50‐kDa form (p50). Mature NF‐κB dimers are sequestered in the cytoplasm by an inhibitor, IκBα, until cellular signals lead to degradation of IκBα and liberation of mature NF‐κB. Only then can mature NF‐κB enter the nucleus and initiate transcription of target genes. (D) RNA Pol II complexes that have stalled, such as at a DNA lesion, are first monoubiquitylated by the E3 ligase NEDD4. A second E3, CUL5ElonginABC, adds K48‐linked polyubiquitin chains to monoubiquitylated RNA Pol II leading to p97/VCP‐dependent removal of the stalled complex from DNA.
