Figure 4. Regulation of transcription by small molecules targeting ubiquitin components.
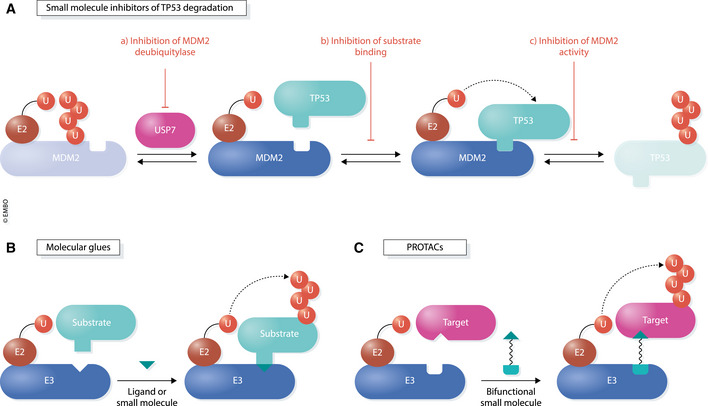
(A) Mechanisms of small molecules that prevent degradation of the transcription factor and tumor suppressor, TP53, by its E3 ubiquitin ligase, MDM2. (a) Inhibitors of the USP7 deubiquitylase lead to increased autoubiquitylation and destabilization of MDM2. (b) Small molecules, such as the Nutlins, block TP53 degradation by disrupting the substrate‐ligase interaction surface. (c) Compounds that block the E3 ligase activity of MDM2, but not substrate binding, also result in accumulation of TP53. (B) Molecular glues are small molecules that alter the surface of proteins, namely an E3 ligase and substrate, to promote their association. These compounds often display low binding affinities for each interactor but simultaneously bind both components to enhance their interaction. (C) PROTACs (proteolysis‐targeting chimeras) are heterobifunctional molecules that consist of two small molecules tethered by a linker. One of the small molecules binds a target protein while the other binds an E3 ligase. In this manner, the cell’s endogenous ubiquitin–proteasome system can be used to rapidly and selectively eliminate any given protein target‐of‐interest.
