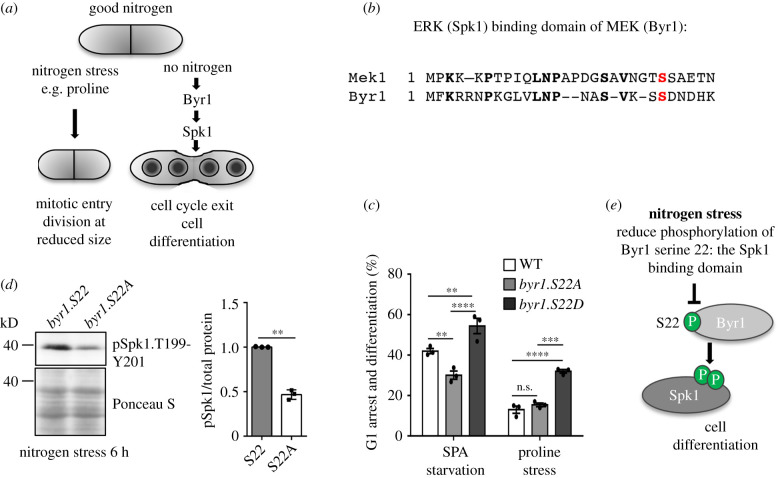
Figure 9.
Reduced phosphorylation of the MAPK-binding motif of Byr1 reduce cell differentiation and sporulation. (a) A schematic that illustrates the two cell fates a cell can adopt after limitation. Following nitrogen stress (a reduction in nitrogen supply or quality), cells advance mitotic commitment and divide at reduced size. After nitrogen starvation (complete removal of a nitrogen source), cells leave the cell cycle and undergo sexual differentiation meiosis and sporulation. (b) An amino acid sequence alignment of S. pombe Byr1 and its human orthologue MEK1. Byr1 serine 22 and MEK1 serine 24 are shown in red. (c) Biological readout of Byr1 activity for phosphorylation site mutants of Byr1 serine 22 upon nitrogen starvation or nitrogen stress: an analysis of the ability to differentiate to form a zygote with 4 spores after 18 h of either nitrogen stress or nitrogen starvation on agar plates, 500 cells were counted. n = 3 with the standard error indicated. **p = 0.01; ***p ≤ 0.001 ****p ≤ 0.0001 (ANOVA, with Tukey's multiple comparison test). (d) Cells were grown in MSL to a cell density of 1.8 × 106 cells ml−1 and then filtered and resuspended in prewarmed MSL minus nitrogen at same density as the original culture. Western blot analysis of total protein extracts from indicated strains after 6 h of nitrogen starvation. Phosphorylation of the Byr1 substrates Spk1 of threonine 199 and tyrosine 201, function as a biochemical readout of Byr1 MAPK kinase activity. n = 3 with the standard error indicated **p = 0.01. (Student's t-test). Ponceau S staining of the western blot membrane has been included as a loading control. (e) Schematic of the role of Byr1.S22 phosphorylation. Phosphorylation at serine 22 is diminished after nitrogen stress in order to stop cells from invoking a full commitment to sexual differentiation until the nitrogen loss is complete.