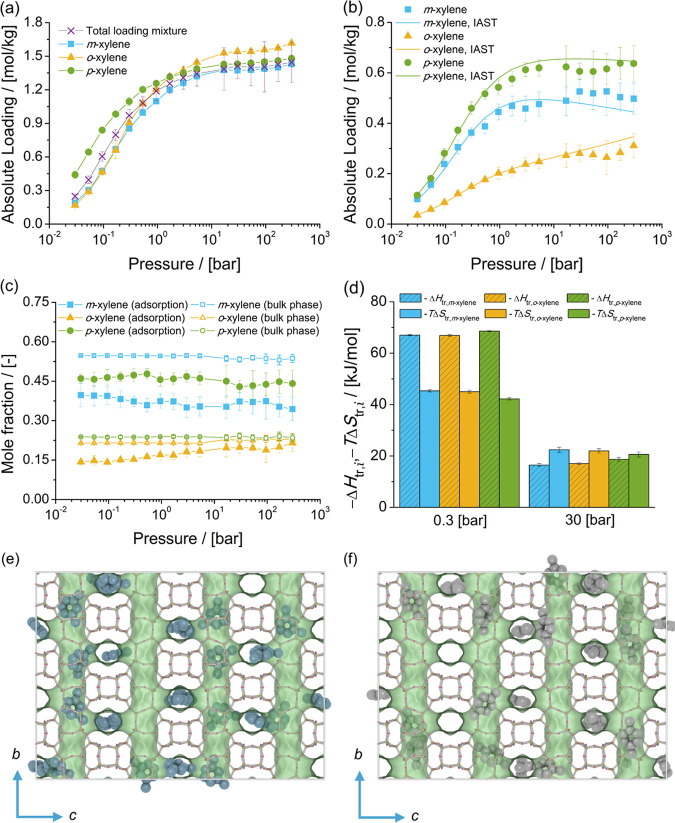
Figure 9.
Adsorption isotherms of xylene isomers as (a) single components (total loading mixture is the sum of the loadings of xylenes from the mixture at chemical equilibrium) and (b) a mixture at chemical equilibrium in BEA-type zeolites at 523 K. (c) Mole fractions of xylene isomers as a function of total pressure for the mixture at chemical equilibrium adsorbed in the BEA-type zeolite and for the bulk phase. The composition in the bulk phase follows from Figure 1a. (d) Changes in enthalpy ΔHtr,i and entropy TΔStr,i at 523 K due to the transfer of xylene i from the fluid-phase mixture at chemical equilibrium to the BEA-type zeolite at 0.3 and 30 bar. Typical snapshot of the simulation of adsorption of the mixture of xylenes in BEA-type zeolites at 523 K and 30 bar showing (e) m-xylene and (f) p-xylene. m-Xylene is shown in blue and p-xylene in gray. The snapshot shows how m-xylene and p-xylene are arranged in the intersection of the channels.