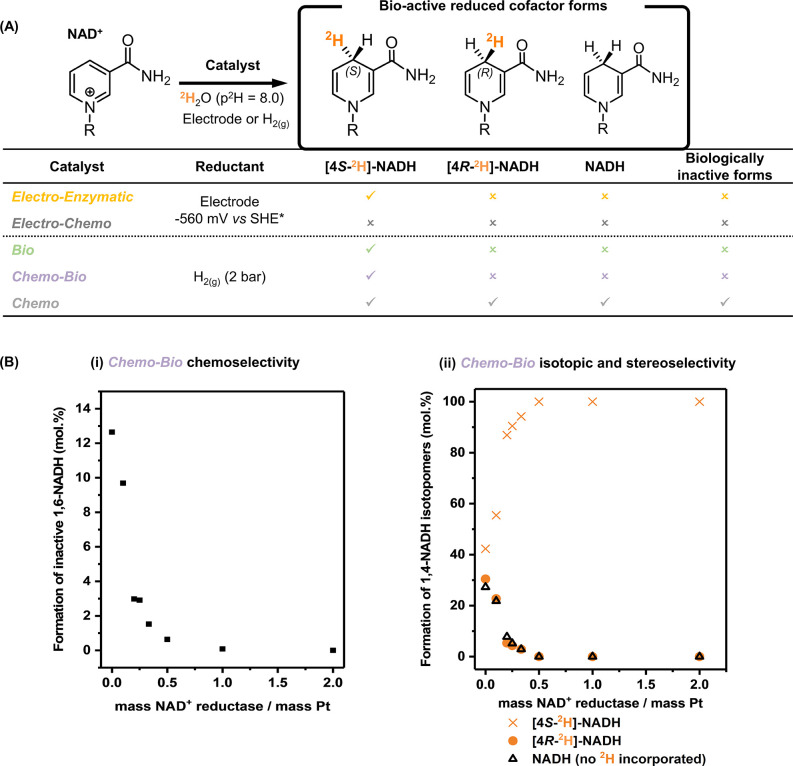
Figure 2.
Selectivity of electrode and H2-driven catalyst systems for generation of [2H]-labeled NADH. (A) Distribution of products formed by each of the Electro-Enzymatic, Electro-Chemo, Bio, Chemo-Bio, and Chemo- catalysts, studied under comparable conditions. The three catalysts containing an NAD+ reductase unit were found to be selective for a single product: [4S-2H]-NADH. Reaction mixtures contained 4 mM NAD+ in 2H2O (p2H 8.0), and the products were analyzed using HPLC and 1H NMR (Figures S5 and S6 in the Supporting Information). For electrode-driven experiments, the electrode was poised at −560 mV vs SHE. For H2-driven reactions the catalysts were operated in H2-saturated solution (2 bar of H2). (B) Experiments to determine the relationship between the enzyme/metal mass ratio and the chemoselectivity, isotopic selectivity, and stereoselectivity of the Chemo-Bio catalyst for making [4S-2H]-NADH from NAD+ under 1H2 gas in 2H2O. Comparisons of selectivity were all made at similar levels of conversion (90–95%). (i) Chemoselectivity (as measured by HPLC): in converting NAD+ to 1,4-NADH, the formation of a biologically inactive side product (1,6-NADH) by Pt/C catalysts was increasingly suppressed by the addition of NAD+ reductase. (ii) Isotopic selectivity and stereoselectivity (as measured by 1H NMR): of the 1,4-NADH formed, a distribution of isotopomers was observed consisting of [4R-2H]-NADH, [4S-2H]-NADH, and unlabeled (no 2H) NADH. Again, increasing the NAD+ reductase loading on the Pt/C catalyst led to the formation of exclusively [4S-2H]-NADH. Note: (*) The Electro-Chemo system was also operated at more negative potentials, giving rise to NADH and biologically inactive forms (see Section S.2.1 in the Supporting Information).