Figure 4.
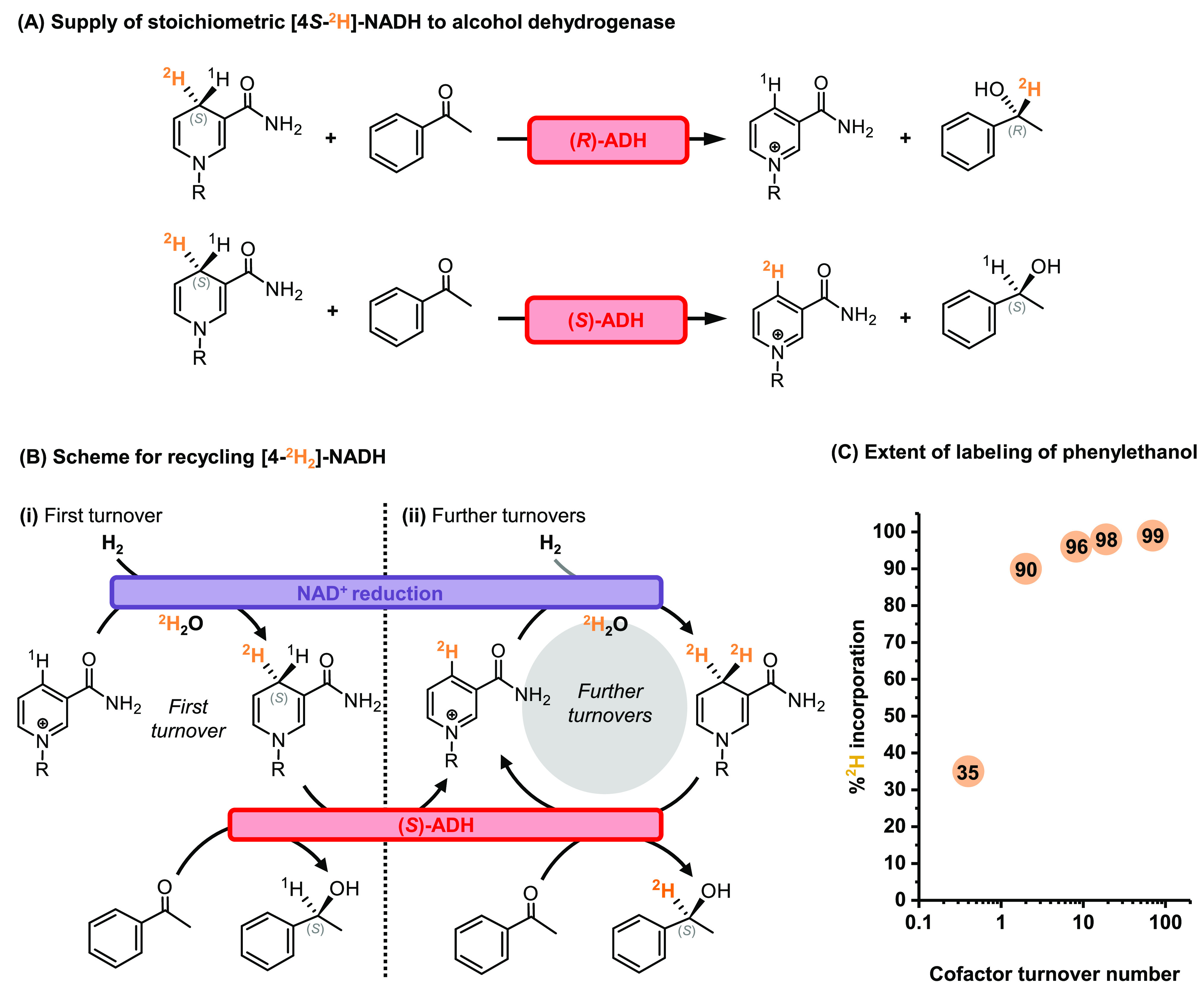
Understanding %2H incorporation of enzymes with opposing facioselectivity. (A) When ADH enzymes with opposite facioselectivity are supplied with [4S-2H]-NADH, xH– is removed from either the S or R face of the cofactor and transferred to the substrate (acetophenone). This leads to generation of either a 2H-labeled product or an unlabeled product (with the 2H label remaining on the oxidized cofactor). (B) If access to the [S-2H]-labeled alcohol is required, the cofactor can be recycled in situ. The first cofactor turnover leads to unlabeled product (i), and further cofactor turnovers proceed via [4-2H2]-NADH and lead to labeled product (ii). (C) The Bio system was used to supply labeled cofactor to (S)-ADH with a range of cofactor/substrate ratios, and the resulting solutions were analyzed by 1H NMR spectroscopy (see Figure S11 in the Supporting Information). Increasing the cofactor turnover number was found to increase 2H incorporation into the phenylethanol product, tending toward 100%. Reactions were conducted on a 0.5 mL scale in 2H5-Tris-2HCl (100 mM, p2H 8.0) with 400 μg of the Bio system as the catalyst and an excess (500 μg) of (S)-ADH being shaken under a steady stream of H2 gas at 20 °C. In all of the reactions the initial loading of acetophenone was kept constant at 10 mM, with 2 vol % 2H6-DMSO as a cosolvent. The starting concentration of NAD+ was then varied from 0.1 to 25 mM to give varying turnover numbers, with all reactions giving conversions >70% after 16 h.
