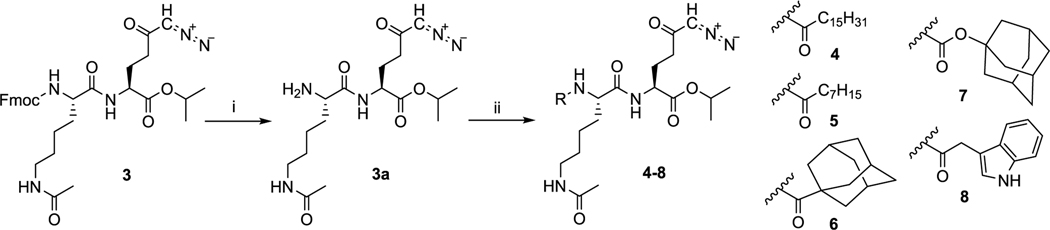
Scheme 2. Deprotection of Intermediate 3 and Synthesis of Prodrugs 4–8a.
aReagents and conditions: (i) diethylamine, DCM, rt, 20 h, 92%; (ii) for 4: palmitic acid, 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate (HATU), DIEA, DCM, 0 °C to rt, 1 h, 63%; for 5: octanoic acid, HATU, DIEA, DCM, 0 °C to rt, 1 h, 65%; for 6: 1-adamantanecarboxylic acid, HATU, DIEA, DCM, 0 °C to rt, 1 h, 82%; for 7: adamantan-1-yl carbonochloridate, triethylamine, DCM, 0 °C to rt, 1 h, 55%; for 8: indolylacetic acid, HATU, DIEA, DCM, 0 °C to rt, 4 h, 53%.