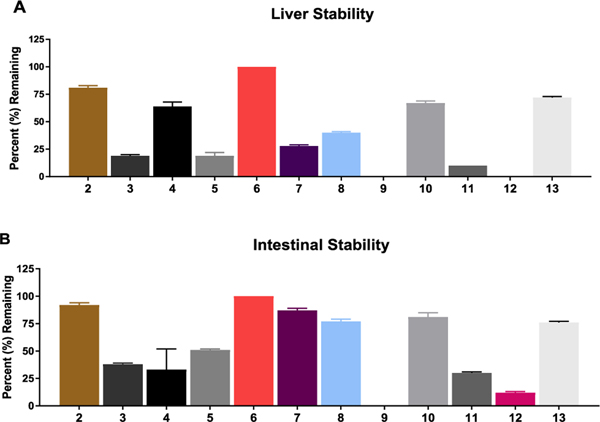
Figure 1.
In vitro metabolic stability studies in swine (A) liver and (B) intestinal homogenates. In liver homogenate, 2, 4, 6, 10, and 13 were found to be stable (>50% remaining at 1 h). In intestinal homogenate, 2, 5, 6, 7, 8, 10, and 13 were stable (>50% remaining at 1 h). The most desirable prodrugs were those stable in plasma, liver, and intestines. On the basis of the plasma (Table 1) and liver and intestinal stability results, 2, 6, 10, and 13 were prioritized into tumor cell partitioning studies.