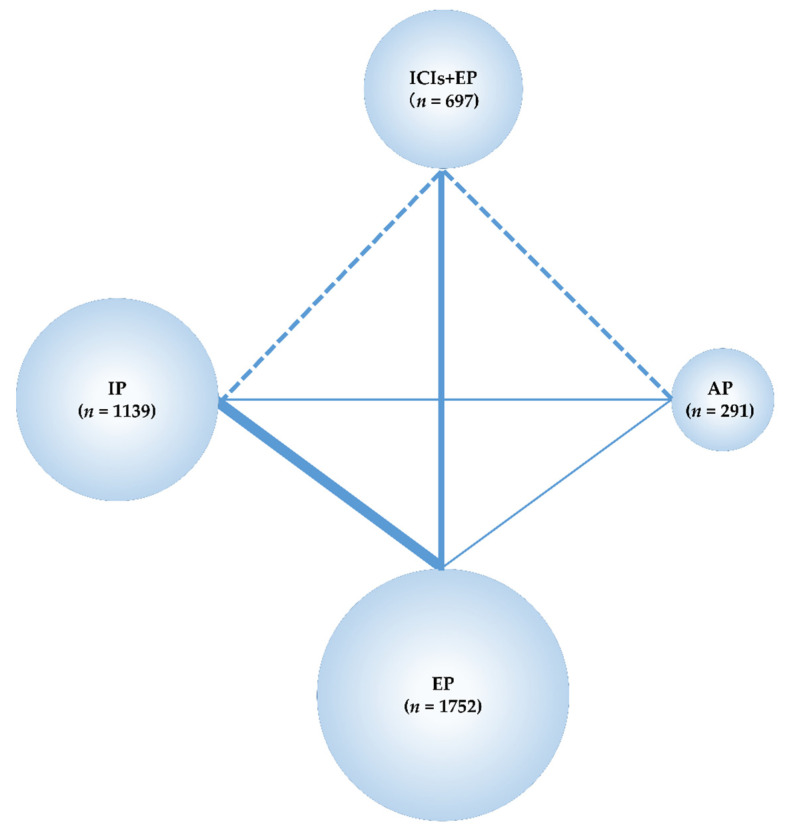
Figure 2.
Network map of four treatment arms of immune checkpoint inhibitors plus platinum–etoposide (ICIs+EP) (combined treated group of pembrolizumab (Pem)+EP, durvalumab (Dur)+EP, and atezolizumab (Atz)+EP), amrubicin (AP), irinotecan (IP), and EP. The randomized controlled trials (RCTs) included in the network meta-analysis (NMA) are indicated by solid lines, and the width of the solid line corresponds to the number of studies included. The dashed line indicates the absence of head-to-head RCTs and that treatment comparisons may be attempted; n, number of patients included in each treatment group. ICIs+EP, immune check point inhibitors plus platinum–etoposide; Pem+EP, pembrolizumab plus platinum–etoposide; Dur+EP, durvalumab plus platinum–etoposide; Atz+EP, atezolizumab plus platinum–etoposide; AP, platinum–amrubicin; IP, platinum–irinotecan; EP, platinum–etoposide; RCT, randomized controlled trial; NMA, network meta-analysis.