FIGURE 5.
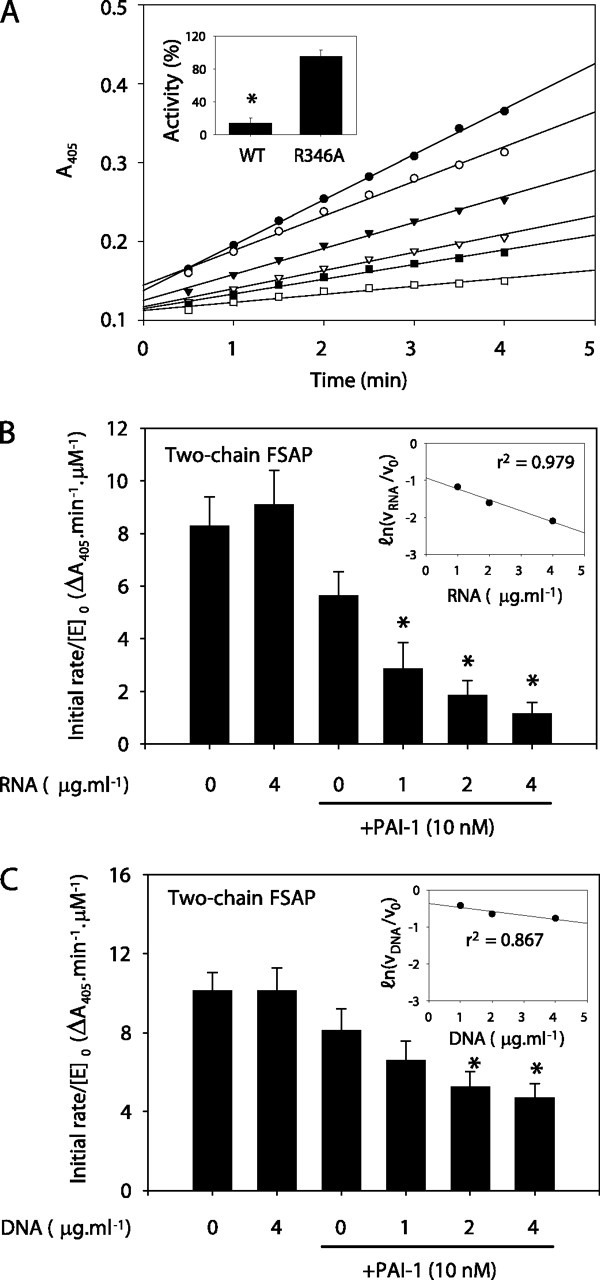
Inhibition of FSAP hydrolytic activity by PAI-1.A, two-chain FSAP (14 nm, by mass) was incubated in the absence (•) or in the presence of active wild-type PAI-1 at a concentration of 10 nm (▾), 14 nm (▿), 19 nm (▪), or 24 nm (□) or in the presence of 19 nm heat-inactivated PAI-1 (○) in 100 μl of TBS buffer. Amidolytic activity was followed as the conversion of the chromogenic substrate S-2288 at 405 nm. A single representative experiment of seven is illustrated. Amidolytic activity of tcFSAP (14 nm, by mass) against S-2288 was evaluated after a 30-min preincubation with WT PAI-1, or PAI-1 R346A (both at 24 nm, by mass) as described above (inset). Data represent mean ± S.D. (n = 7). *, p < 0.001 between PAI-1 WT- and R346A-treated tcFSAP. The effect of RNA (B) or DNA (C) on the inhibition of tcFSAP by PAI-1 was also assessed. In both instances, the linear dependence of PAI-1 inhibitory capacity on RNA and DNA concentration was assessed by a semilog plot of residual enzyme activity versus RNA or DNA concentration (insets). The correlation coefficient, r2, is indicated in the insets. Data represent mean ± S.D. (n = 7). B, *, p < 0.001 versus PAI-1 in the absence of nucleic acids.
