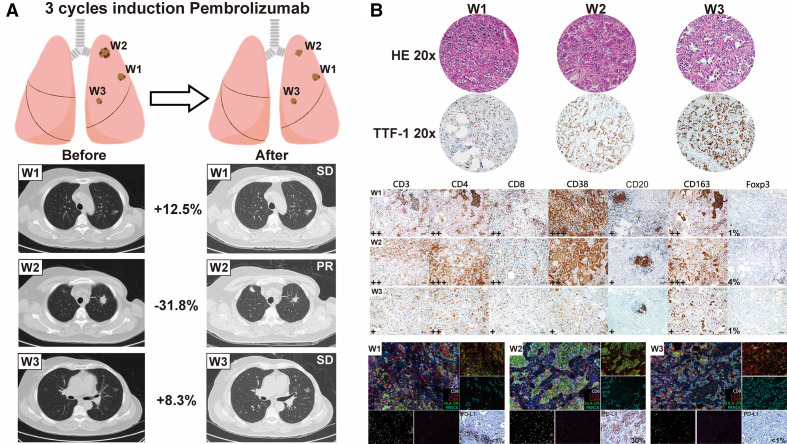
Figure 1.
Patient clinical and pathological evaluation following neoadjuvant immunotherapy. (A) Radiological evaluation of three resected nodules after three cycles of neoadjuvant immunotherapy showing notable tumor shrinkage of the solid nodule; however, the other nodules remained stable. (B) Comprehensive pathological evaluation integrated with mIHC of three surgically resected nodules. The extent of staining and the percentage of Foxp3-positive cells with nucleus staining are listed on the lower left corner of each slide. mIHC and PD-L1 images using the Dako 22C3 assay of nodules are obtained. mIHC panels are displayed in color on the lower right corner of the IHC slides: CD8 (white), CD56 (purple), CD68 (red), HLA-DR (green); PANCK (cyan). The expression level of PD-L1 is listed on the lower right corner of IHC slides. CNV, copy number variation; HLA, human leukocyte antigen; IC, immune cell: mIHC, multiplex immunohistochemistry; PD-L1, programmed death ligand 1; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; TMB, tumor mutation burden; TTF-1, thyroid transcription factor 1.