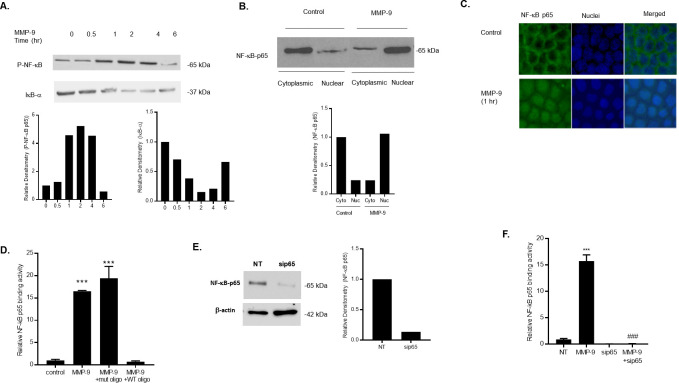
Fig 2. Effect of MMP-9 on NF-κB p65 activation in Caco-2 monolayers.
(A) Phospho-NF-κB p65 and IκB-α expression were determined in filter-grown Caco-2 monolayers treated with MMP-9 (400 ng/ml) for increasing time periods (0–6 hrs). (B) NF-κB p65 expression in the cytoplasmic and nuclear fractions was assayed by Western blot analysis after MMP-9 treatment (1-hr experimental period). (C) NF-κB p65 cytoplasmic-to-nuclear translocation was determined by immunostaining. (Yellow, NF-kB p65; Blue, DAPI (nuclei)). Original magnification, ×40. (D) NF-κB p65 binding to the oligonucleotide probe containing the κB-binding site was determined by ELISA-binding assay. MMP-9 caused a significant increase in NF-κB p65 binding (1-hr experimental period). The oligonucleotide containing a mutated NF-κB-binding (mut) motif did not inhibit the NF-κB p65 binding to the DNA probe; however, the addition of wild-type (WT) oligonucleotide containing the consensus NF-κB p65-binding site as a competitive inhibitor prevented the binding of NF-κB. *** P < 0.001 vs control. (E) NF-κB p65 siRNA transfection resulted in a marked depletion in NF-κB p65 protein expression. Caco-2 monolayers were transfected with NF-κB p65 siRNA for a 72-hr time period, (NT; not-target siRNA). (F) NF-κB p65 siRNA knock-down prevented the MMP-9 induced increase in NF-κB p65 binding to the oligonucleotide probe containing the κB-binding site. *** P < 0.0001 vs control; ### P < 0.001 vs MMP-9 treatment.