Abstract
The distributed pharmaceutical analysis laboratory (DPAL) is a collaboration between 30 academic institutions around the world, whose goal is to determine the quality of medicines collected from partner organizations in low- and middle-income countries (LMICs). Institutions complete system suitability for a high-performance liquid chromatography (HPLC) system using United States Pharmacopeia (USP)-traceable reference standards, and are then approved to analyze batches of samples that are collected in LMICs by covert shoppers. Open Science Framework (OSF) allows DPAL participants access to resources for the program including an HPLC methodology manual, a wiki with HPLC troubleshooting information, detailed checklists and Excel templates for system suitability and sample assay, as well as steps for reporting results. Participants incorporate the DPAL program into their academic curriculum as undergraduate research or via lab activities for analytical chemistry or instrumental analysis courses. Over a thousand samples have been analyzed through DPAL in the last three years, and 168 samples with quality problems have been discovered, including falsified acetaminophen, adulterated amoxicillin-clavulanate and doxycycline, and substandard losartan. These quality problems are reported to the medicine regulatory agencies in the countries of origin and the WHO Rapid Alert System for further action. This real-world program gives students a hands-on opportunity to see the importance of analytical metrics taught in the classroom.
Keywords: Second-Year Undergraduate, Analytical Chemistry, Hands-On Learning, Inquiry-Based Learning, Applications of Chemistry, Pharmaceuticals, Instrumental Methods, HPLC, Quantitative Analysis, Undergraduate Research
Graphical Abstract
INTRODUCTION
The use of real-world applications in scientific teaching can be greatly beneficial for students. Learning is enhanced when the discussion shows how the topic matter can solve problems that are familiar to students.1,2 When their research or class topic shows up on the evening news, it powerfully illustrates to students that science does, indeed, have relevance to peoples’ lives.3,4,5
Substandard and falsified pharmaceuticals are a world-wide problem, with disproportionate impact in low-and middle income countries (LMICs). The World Health Organization (WHO) reported that one in ten medical products being sold in LMICs are substandard or falsified.6 Substandard pharmaceuticals can cause drug resistance, increased mortality and morbidity, and lead to a loss of confidence in the healthcare system.7 Lack of efficacy, harmful side effects, and even death can arise from falsified pharmaceuticals that have been adulterated with inert fillers or substitute active pharmaceutical ingredients (APIs).8
Most pharmacopeia assays rely on high performance liquid chromatography (HPLC), which is a scarce resource in LMICs. An HPLC costs tens of thousands of dollars not including operational costs such as purified solvents, analytical grade balances and glassware, columns, and pharmaceutical standards. Additionally, complex infrastructure such as consistent power, reliable transportation of supplies, availability of trained operators and resources for maintenance and repairs, and proof of a high level of training and documentation are required for sustainable operation. These costs are magnified by the extensive assays required by the full monograph procedure and the fact that if 10% of the drugs in a market are substandard or falsified, nine out of ten drugs are NOT substandard or falsified. The cost of detecting each bad quality product must include the cost of analyzing multiple good quality products. For this reason, routine analysis of medicine quality is severely limited in many LMICs.
One way to tackle a large-scale project such as pharmaceutical post-market surveillance is using crowdsourcing or citizen science. Citizen science refers to a participative way of running scientific research projects, done mostly through the Internet for data collecting, processing, and analysis.9,10 This model allows for a monitoring system that cannot be attainable by individual or government entities alone.11 We founded the distributed pharmaceutical analysis lab (DPAL) after realizing that we had too many samples for our lab to analyze in a meaningful timeframe. By partnering with other institutions, we were able to continue collecting hundreds of samples to gather a better understanding of medicine quality through the region while also speeding analysis.
The distributed pharmaceutical analysis lab project aims to make analytical capacity at colleges and universities around the world available to carry out preliminary HPLC tests of medicines purchased in LMICs.12 Our goal in DPAL is to allow regulatory agencies in LMICs to focus their analytical resources on the worst quality products. While other analytical chemistry experiments have been developed to allow students to study pharmaceuticals,13,14,15 the focus of DPAL is on providing as robust an operational environment as possible in an academic setting. Thus, the program emphasizes standard operating procedures, record keeping, verification of system suitability, and quality assurance/control (QA/QC) practices. By placing these in the natural context of an important real-world assay, we aim to help students see why analytical chemists care about reliability of assays and how their own careful work can contribute to finding and thwarting drug counterfeiters.
MATERIALS
Sample Collection and Intake Protocol
Details of sample collection and intake were described in previous work.12 Briefly, covert shoppers collect samples of pharmaceuticals from small medicine shops in cities and towns across Western Kenya which are then brought to Moi Teaching and Referral Hospital (MTRH), where the sample metadata (brand, expiration date, lot number, etc.) are transcribed onto a spreadsheet prior to shipping to the University of Notre Dame (UND). Permission was obtained from the MTRH Institutional Research & Ethics Committee (protocol # 000836) for the collection and shipment of samples. 16 In 2016, 2017, 2018, and 2019, 1,300, 150, 200, and 667 samples were collected respectively.
All samples are assigned a Notre Dame identification number (NDID), which is used for sample reporting. A brief physical examination of each sample is conducted to identify packaging issues that could lead to product degradation, or which indicate poor manufacturing. After sample intake, samples are stored in a 4°C cold room until they are sent for analysis.
DPAL Participant Requirements and Costs
Academic institutions that participate in DPAL are required to have the basic instrumentation required for pharmaceutical analysis including: a properly calibrated analytical balance, a high-performance liquid chromatograph with ultraviolet, photodiode array, or mass spectrometer detector capable of collecting and analyzing data. The cost of columns, analytical standards, and HPLC solvents for many pharmaceutical assays is comparable to the costs for other undergraduate HPLC experiments, so the DPAL experiments fit into existing budgets for undergraduate analytical or instrumental analysis courses.12 In some cases, where funds are limited, the DPAL project has been able to provide reference materials and access to monographs through a partnership with the US Pharmacopeial Convention (USP).
Open Science Framework (OSF)
After participants have expressed interest in joining the Distributed Pharmaceutical Analysis Lab, they are given access to the DPAL Open Science Framework (OSF) project.17 By joining the DPAL OSF site, participants must agree to abide by DPAL policies regarding data security, integrity and publishing. OSF is free to use and only requires an Open Researcher and Contributor ID (ORCID) to register.18 All participants are given access to the main DPAL site where they can download the HPLC Methodology Manual, found in the supporting information. The Methodology Manual is a continuously updated document that defines the standard operating procedures for carrying out pharmaceutical assays. Also available are checklists for system suitability, Excel spreadsheets formatted for one- and two-drug products, a wiki for exchanging HPLC troubleshooting tips, and other information that participants can download and use freely.12
Each participant school has its own component OSF site, which contains their analytical information, data, and a wiki. The faculty advisor for the participant school can load the Methodology Manual and Excel templates to the component site and give other faculty members and students access. Students can download the template files and upload their data, scan and share their notebook pages or make wiki entries.12 OSF allows users to create a snapshot of their site with its own DOI; this registered OSF site can be used to archive the raw data and calculated results for a publication or presentation.
Legal Considerations
DPAL is not a certified pharmaceutical analysis laboratory; participants perform single-tablet API assays and do not conduct full compendial testing on pharmaceutical products. This means that the data produced cannot be used in a legal context to certify a medicine’s quality. The goal of the DPAL is to report suspicious samples to medicine regulatory agencies, so they can conduct the compendial analysis. DPAL provides templates to participants to record their HPLC method, system suitability results, and sample analysis results to ensure uniform procedures and calculations are conducted so the data can be traced back and checked for error.12 The HPLC Methodology Manual explicitly states the expectations for quality and data security and addressed procedural protocols.12 Legal considerations of pharmaceutical analysis are discussed to ensure that all participants fully understand how to correctly report and present DPAL results.
System Suitability Requirements
After choosing an analyte whose assay is based on USP methodology, the DPAL participant must demonstrate system suitability through a series of tests laid out in USP <1225> and USP <1226>. These tests determine whether the methodology and the instrument are working properly for analysis of a particular pharmaceutical. The results of the system suitability tests must be within acceptable limits defined in the Methodology Manual to be considered valid. Participants must show their results in a provided Excel template to prove that they have met the qualifications for each experiment. The DPAL program requires that participants successfully complete all the required system suitability tests and submit the results for review before samples from LMICs are sent for analysis.12
System suitability experiments are separated into two parts. Part One is based on samples prepared using a reference standard and includes:
Keeping a record of peak metrics including asymmetry and theoretical plates with sample chromatograms.
Establishing a control chart that includes information about the peak shape, retention time, resolution, and integrated intensity. This document is not only used as a record of activity for each day, but functions as a diagnostic reference when issues arise.
Instrument precision is tested by the injection of one sample six consecutive times. The relative standard deviation of the integrated intensity for each run must be less than 2%.
To meet the linearity requirement for system suitability, the y intercept must fall within the error of zero, and the R2 value should be greater than 0.98.
The accuracy and range experiment demonstrates that the method can accurately analyze various sample concentrations along the defined linear range. To evaluate the accuracy and range, four solutions are prepared: an overdosed sample, a normal sample, a deficient sample, and a blank which contains no API. Each of these solutions are run in triplicate and the measured concentrations of the samples should be within 2% of their true concentration.
Limit of detection (LOD) is calculated to determine the lowest quantity detectable that is significantly different from zero. The experiment is conducted via the slope-standard deviation method, with the standard deviation generated by running six samples at concentrations 2–3 times the expected LOD as estimated from the linearity plot. A chromatogram must be collected at the calculated LOD concentration to confirm detection.
Part Two of the system suitability is conducted using real dosage forms supplied by UND and includes:
For the spike recovery experiment, a pharmaceutical dosage form is used to make the sample solution. An aliquot of the sample solution is prepared with an additional 30% spike of API standard. The spike is determined by comparing the sample assay to the spiked sample assay. An acceptable spike recovery is between 90–110% of the spike.
Specificity is completed like spike recovery but with a complex matrix, such as one that contains degradation products of the API. An expired sample may be used, or one that has been thermally or chemically degraded. The samples are run per the spike-recovery method to calculate percent recovery. An acceptable spike recovery is between 90–110% of the spike.
After the participant institution meets the system suitability requirements, they can analyze pharmaceutical samples from LMICs countries. Before each day’s sample analysis, five injections of an external calibration standard are analyzed to check precision (alternatively five solutions are prepared to generate a calibration curve which must meet the linearity requirements for system suitability), and this calibration standard is rechecked every five samples to be sure the integration stays within the 2% RSD limit. If the calibration check fails, the five preceding samples must be re-run. For each assay, the mass of the capsule or tablet and the stated API content are recorded and used as for determining the total amount of active pharmaceutical ingredient in the sample. If the pill does not meet pharmacopeia specifications found in an official monograph (generally 90–110 or 120% of stated API content, depending on the monograph) the sample is re-analyzed to confirm the result. If the sample still fails to meet quality specifications, then two additional capsules or tablets are assayed. Samples that are found to be substandard are analyzed at the University of Notre Dame for additional testing, and results are reported to the country’s regulatory agency and the WHO Rapid Alert program.
RESULTS
The DPAL project started in 2014 and expanded significantly in 2018 after presentations on the project at the BCCE meeting.19 There are currently 29 institutions in DPAL. Some of these schools incorporate the DPAL program as an undergraduate research project or chemistry club activity, others as part of instrumental analysis or analytical chemistry courses. 15 of the institutions have completed system suitability for at least one drug, and 11 have reported assays for samples. Figure 1 shows the participating institutions, their target pharmaceuticals, and their status within the program.
Figure 1.
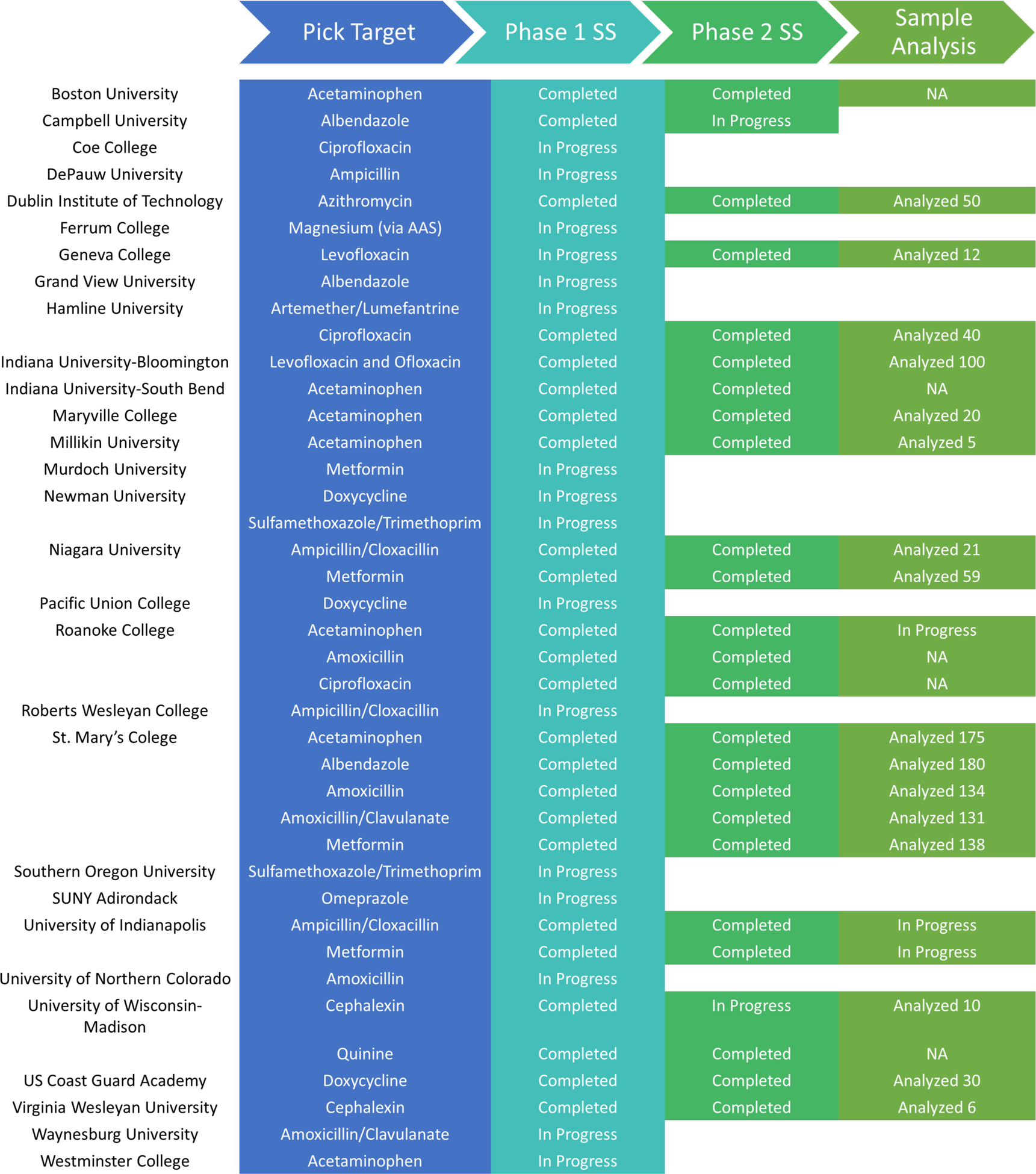
DPAL Participants.
DPAL participants were surveyed about the program structure and outcomes from their participation.Eleven participants who had completed at least phase 1 of system suitability testing responded to the survey. Most of the participants (73%) were either the only analytical chemist in their department, or had one other analytical colleague. Instructor support and the connections with analytical chemists at other DPAL schools were viewed as a positive feature, “DPAL makes it easier on me as a supervisor because there is a clear path to follow for every student and they have to show proficiency at each step before they can go on to the next step - not unlike working at a commercial analysis facility.” “Although we are independently collecting and analyzing our own samples, we take advantage of the validated HPLC methods developed by other groups.”
Over 90% of participants agreed or strongly agreed that DPAL engages students and that it is relevant to instrumental analysis topics. Features that advisors felt contributed to student engagement included the humanitarian application—as one respondent put it, “The analysis was implemented with a freshmen honors chemistry class. They could not believe that they were doing lab work that really mattered.” The tie-ins to other instrumentation topics were also seen as motivating, as one respondent noted “Doxycycline capsule contents varied widely. Our students are planning to use FT-IR and RAMAN to analyze the differences associated with each of them. We will also look at the carbon and proton NMR for further analysis.” Advisors noted that although the instrumental techniques were usually straightforward, students gained a deep understanding of instrumental quality control. They felt that this feature of the project built skills that are needed by industrial chemists. “It transforms mundane and boring technical work into the important context that these students would face if they worked in a pharmaceutical lab, and having that reality during the learning event makes the students much more engaged and committed to doing good work.”
The DPAL projects have two main stages: system suitability, followed by sample analysis. Sample analysis was viewed as slightly easier to incorporate into the analytical lab courses, mostly because system suitability involves a larger variety of tasks and a greater number of potential problems that could arise. “…sample analysis is a simpler SOP and was easy to implement in the teaching laboratory.” “System suitability requires greater dedication and time commitment along with strong record-keeping and so I think it better matches with undergraduate research.” “The system suitability process took one and a half semesters to troubleshoot and perform. We moved at the speed of my one research student who is signed up for six hours a week.” Other participants worked system suitability into a lab class, “The mathematics required for determining and understanding LOD or standard deviation in conjunction with the HPLC-UV/Vis is a great way to show the importance of the instrument to an analytical class.” All participants agreed that both system suitability and sample analysis were suitable for undergraduate research projects; 42 students have participated in this form of the DPAL program. 36% of the respondents implemented at least one of the DPAL tasks in analytical or instrumental analysis courses as well, and 171 students participated in this form of the DPAL program. Some respondents felt that sample analysis was too limited as the basis for an undergraduate thesis, but that the projects could be expanded by including other instrumental techniques or analysis methods, “The ability to participate in this program early has allowed students to gain a better understanding of analytical procedures. Continued participation also allowed them to think of other analysis techniques (degradation study, FT-IR or RAMAN analysis).” A total of 68 poster presentations, 38 oral presentations, and 28 senior theses or capstone reports have been completed by undergraduates throughout the program. Most of these were internal presentations, but there have been nine national or international poster presentations and three national or international oral presentations by DPAL participants.
Over one thousand samples have been analyzed through DPAL. A total of 168 samples failed HPLC assay; this level of failing samples is consistent with estimates for the prevalence of substandard and falsified (SF) pharmaceuticals in the countries where the samples were collected.20 Failing samples included falsified acetaminophen tablets, losartan pills with unacceptable pill-to-pill variation in API content, falsified doxycycline capsules, substandard and falsified amoxicillin capsules,21 substandard amoxicillin/clavulanic acid tablets, and degraded injectable ceftriaxone.22 A report of the findings is sent as quickly as possible to the drug regulatory agency and partner organizations in the country where the samples were collected; products with less than 50% API content are also reported to the WHO Rapid Alert system.
Products that fail quality standards undergo additional testing at both UND and the DPAL partner school to confirm the HPLC findings and gain insight into the root cause of the failure. These root cause investigations extend the instrumental analysis methods used by students and are also a useful contribution to the reports to regulators, as they may guide possible regulatory responses.
One hundred and ten samples of acetaminophen were analyzed with only two failing to meet quality standards. One sample was deficient in API content and the other did not contain any active ingredient. The falsified sample was found to consist of 40% starch and 60% inorganic material. Figure 2A shows the paper analytical device (PAD)23 screening that detected the starch and noted the absence of acetaminophen. Figure 2B shows the ultraviolet (UV) spectrum that identified the dye used to color the tablet as Sunset Yellow FCF. Figure 2C shows the X-ray photoelectron (XPS) spectra that suggested the presence of talc and aluminum oxide as the inorganic components left over after extraction. This sample illustrates the pervasiveness of drug falsification—even a low-cost product like generic paracetamol is the target of counterfeiters.
Figure 2. Falsified Sample of Acetaminophen.
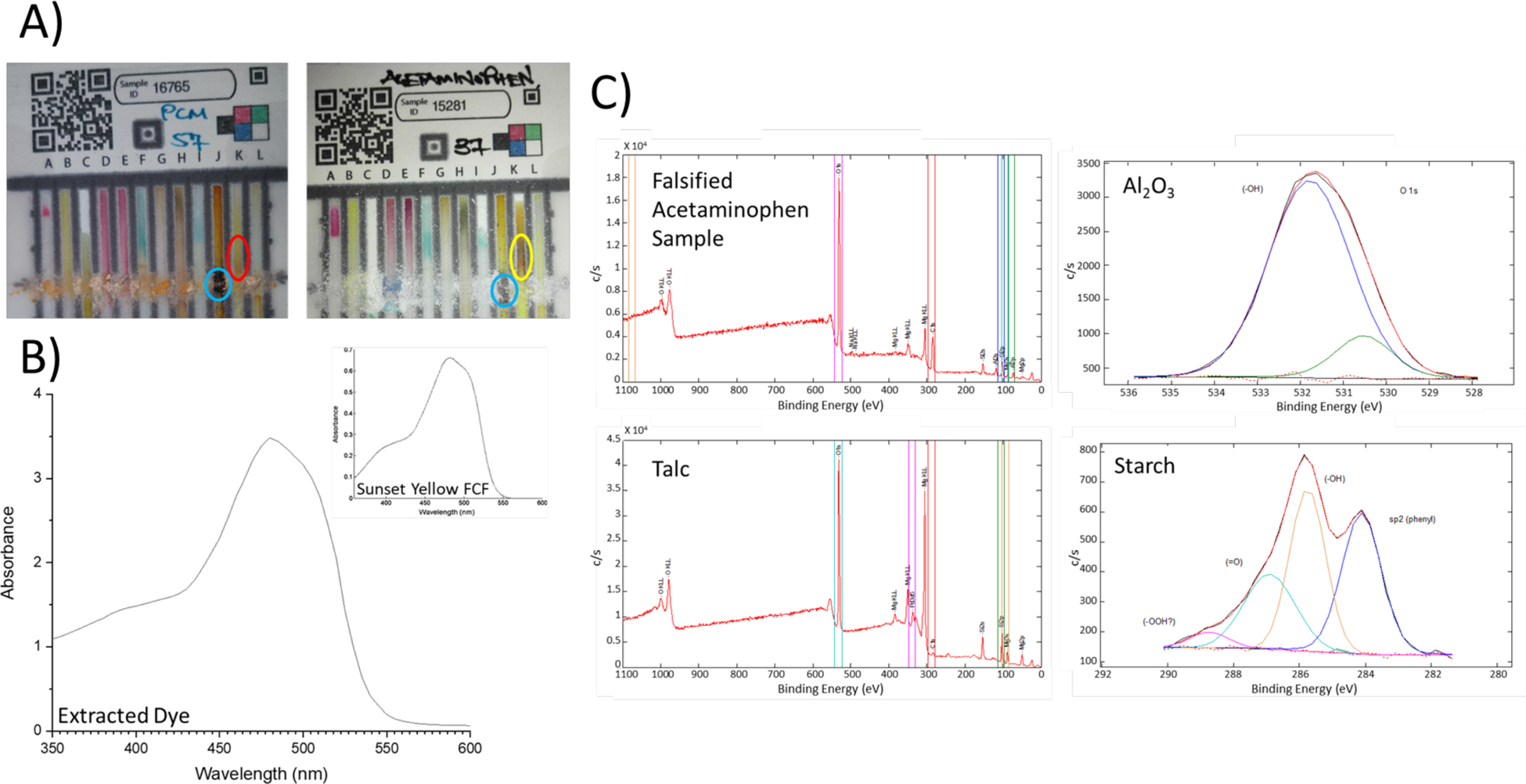
A) Left PAD is the suspicious sample that did not turn Lane K brown (red circle) as shown in the PAD on the right (yellow circle). Both PADs detected starch (blue circles). B) Extracted dye in chloroform from the suspicious sample with an insert of Sunset Yellow FCF used to confirm the identity. C) Left XPS spectra show the comparison of the suspicious sample to talc and the right show the detection of aluminum oxide and starch.
Thirty-eight losartan samples were assayed. Over one-third (39.5%) of the blister packs showed pill heterogeneity issues, where one or more of the tablets within the blister pack would fail the assay but a neighboring tablet would pass the assay. This quality issue was found throughout all samples and not linked to any specific brand. An example of this heterogeneity is shown in Table 1. This type of failure represents a possible failure of good manufacturing process (GMP), so the country regulatory authorities receive a report and these brands may receive more scrutiny in future rounds of testing.
Table 1.
HPLC Results Demonstrating Quality Issues with Losartan Samples.
| Sample | API Present, % | Result |
|---|---|---|
| Pill Heterogeneity Issuea | ||
| Pill One | 70.2 | Fail |
| Pill One Retest | 70.0 | Fail |
| Pill Two | 75.1 | Fail |
| Pill Three | 90.5 | Pass |
| Substandard Quality Issueb | ||
| Pill One | 82.6 | Fail |
| Pill One Retest | 86.2 | Fail |
| Pill Two | 83.1 | Fail |
| Pill Three | 81.7 | Fail |
Two tablets failing analysis.
All three tablets failing analysis.
Forty-nine samples of doxycycline were analyzed and 71.4% of them were found to be falsified products composed of 50% API and 50% talc. This product was reported immediately to the country drug regulatory agency and to the WHO Rapid Alert system. Figure 3 shows the attenuated total reflectance-Fourier-transform infrared (ATR-FTIR) spectra for a standard of doxycycline, a good quality dosage form, a falsified product, and pure talc. The strong band at just below 1000 cm−1 is from the Si-O stretching modes for silicate groups in the talc. The venerable technique of gravimetry was used to determine the talc percentage after extensive washing with methanol to remove doxycycline.
Figure 3.
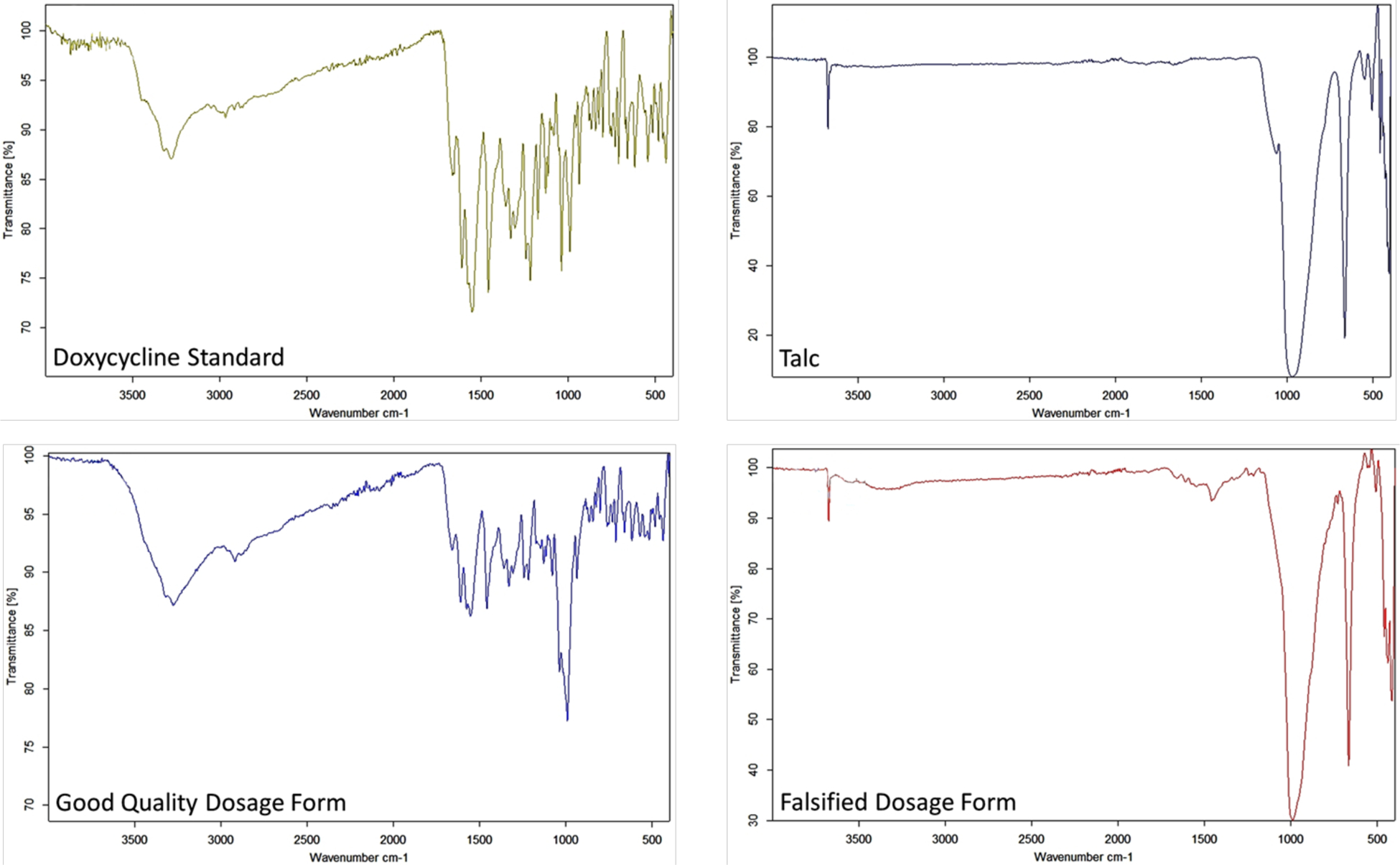
ATR-FTIR Spectra for Doxycycline Dosage Forms.
DISCUSSION
The multifaceted aspect of the Distributed Pharmaceutical Analysis Laboratory makes it an ideal project for the undergraduate analytical curriculum.12 Completion of the system suitability components can take 1–2 semesters which allows them to serve as a student research project focused on HPLC troubleshooting and analytical metrics prior to conducting sample analysis. Alternatively, completion of system suitability can be conducted in an analytical chemistry or instrumental analysis lab, with different students being responsible for different components of suitability testing. In the past, we have also had instructors or their TA’s complete system suitability prior to students in the class so they can focus on sample analysis by only replicating parts of the system suitability requirements with the entire class.
The effectiveness of the DPAL program is limited by several logistic factors, largely dependent on communication.12 Participants must complete the phase one portion of system suitability using reference-grade standards which is reviewed by DPAL program coordinators prior to sending samples from completion of the phase two portion of system suitability. Upon completion of sample analysis, substandard samples are further tested at Notre Dame to determine composition and a final report is prepared by the DPAL participant and DPAL program coordinators for the medicine regulatory agencies to review. Using Open Science Framework has helped expedite the data sharing process for more rapid turnaround from system suitability completion to sample analysis.
CONCLUSION
Poor quality pharmaceuticals are a problem in many LMICs due to the cost of conducting instrumental analysis required for effective post-market surveillance. The Distributed Pharmaceutical Analysis Laboratory engages academic institutions around the world that can conduct assays of medicine collected in LMICs via HPLC. The tests for system suitability and metrics for conducting sample analysis required from DPAL participants fit well into instrumental analysis and analytical chemistry lab courses, and can also be carried out via undergraduate research. To assure that the sample analysis results are accurate, DPAL participants must complete both phases of system suitability which is reviewed by the DPAL program coordinators before analyzing samples. Open Science Framework is used for recording raw data, calculations, and summary reports between DPAL coordinators and participants. OSF houses templates, HPLC methods, and troubleshooting tips accessible by all DPAL participants. Further development of the program will focus on connecting DPAL participants to collaborators in LMICs for program growth and impact.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank all our DPAL participants: Rosina Georgiadis (Boston University), Jordan Womick (Campbell University), Martin St. Clair (Coe College), Richard Martoglio (DePauw University), Patrice Behan (Dublin Institute of Technology), Claire McDonnell (Dublin Institute of Technology), Laura Grochowski (Ferrum College), Maria Puccio (Ferrum College), Rodney Austin (Geneva College), Corbin Zea (Grand View University), Deanna O’Donnell (Hamline University), Rita Majerle (Hamline University), Jill Robinson (Indiana University-Bloomington), Grace Muna (Indiana University-South Bend), Mary Turner (Maryville College), Kyle Knust (Milikin University), Damian Laird (Murdoch University), Ryan Huschka (Newman University), Robyn Goacher (Niagara University), Robert Wilson (Pacific Union College), Gary Hollis (Roanoke College), Tim Johann (Roanoke College), Skip Brenzovich (Roanoke College), Jason Taylor (Roberts Wesleyan College), Toni Barstis (Saint Mary’s College), Steven Petrovic (Southern Oregon University), Christine O’Connor (SUNY Adirondack), Levi Mielke (University of Indianapolis), Corina Brown (University of Northern Colorado), Pam Doolittle (University of Wisconsin-Madison), Jacob Loman (US Coast Guard Academy), Mike Persun (US Coast Guard Academy), Maury Howard (Virginia Wesleyan University), Joyce Easter (Virginia Wesleyan University), Heidi Fletcher (Waynesburg University), and Erin Wilson (Westminster College) along with their many students. We acknowledge generous donations by the United States Pharmacopeia (www.usp.org) of reference standards and for the access to the National Formulary of pharmaceutical monographs provided to DPAL members. The authors would like to acknowledge Nicholas M. Myers for his assistance in developing the DPAL program. The authors would like to acknowledge Mercy Maina and Phelix Were for maintaining the metadata and inventory of samples collected in Western Kenya. Funding for core services was provided through a grant from the Indiana Clinical and Translational Sciences Institute funded, in part by Grant Number UL1TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support for sample collection was provided in 2018–2020 by an NSF award, grant number NSF-CMMI-1842369.
Footnotes
Supporting Information
The Supporting Information is available on the ACS Publications website at DOI: 10.1021/acs.jchemed.xrefX.
HPLC Methodology Manual (PDF) System Suitability Templates (XLXS) Sample Analysis Templates (XLXS)
REFERENCES
- 1.Gallagher JJ Teaching for Understanding and Application of Science Knowledge. Sch. Sci. Math, 2000, 100 (6), 310–318. [Google Scholar]
- 2.Fortus D; Krajcik J; Dershimer RC; Marx RW; Mamlok-Naaman R Design-based science and real-word problem-solving. Internat. J. Sci. Ed, 2005, 27(7), 855–879. [Google Scholar]
- 3.Strand KJ Community-Based Research as Pedagogy. Mich. J. Comm. Serv. Learn, 2000, 85–96. [Google Scholar]
- 4.Piranty S Coronavirus fuels a surge in fake medicines. BBC World Service, 2020. https://www.bbc.com/news/health-52201077 (accessed 2020–09-09).
- 5.Hirschler B Tens of thousands dying from $30 billion fake durgs trade, WHO says. Health News, Reuters, 2017. https://www.reuters.com/article/us-pharmaceuticals-fakes/tens-of-thousands-dying-from-30-billion-fake-drugs-trade-who-says-idUSKBN1DS1XJ (accessed 2020–09-09).
- 6.World Health Organization. WHO Global Surveillance and Monitoring System for Substandard and Falsified Medical Products. 2017. License: CC BY-NC-SA 3.0 IGO.
- 7.Newton PN; Green M; Fernandez FM Impact of poor-quality medicines in the ‘developing’ world. Trends Pharmacol. Sci 2010, 31, 99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. WHO Medical Product Alert No. 4/2015: Adverse reactions caused by falsified Diazepam in Central Africa. 2015, Ref. RHT/SAV/MD/4/2015
- 9.Prats López M; Soekijad M; Berends H; Huysman M A Knowledge Perspective on Quality in Complex Citizen Science. Citizen Science: Theory and Practice. 2020, 5(1), 15. [Google Scholar]
- 10.Aristeidou M; Herodotou C Online Citizen Science: A Systematic Review of Efforts on Learning and Scientific Literacy. Citizen Science: Theory and Practice. 2020, 5(1), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roger E; Turak E; Tegart P Adopting Citizen Science as a Toll to Enhance Monitoring for an Environment Agency.Citizen Science: Theory and Practice. 2019, 4(1), 35. [Google Scholar]
- 12.Bliese SL; Berta M; Myers NM; Lieberman M Distributed Pharmaceutical Analysis Laboratory (DPAL): Citizen Scientists Tackle a Global Problem in Mobilizing chemistry expertise to solve humanitarian problems, 2017, ACS Symposium Series; 1267/1268, 117–128. [Google Scholar]
- 13.Fakayode SO Purity Analysis of the Pharmaceuticals Naproxen and Propranolol: A Guided-Inquiry Laboratory Experiment in the Analytical Chemistry Laboratory. J. Chem. Educ, 2015, 92 (1), 157–162. [Google Scholar]
- 14.Zakrzewski R; Skowron M; Ciesielski W; Rembisz Z Spectrophotometric Determination of 6-Propyl-2-thiouracil in Pharmaceutical Formulations Based on Prussian Blue Complex Formation: An Undergraduate Instrumental Analysis Laboratory Experiment. J. Chem. Educ, 2016, 93 (1), 182–185. [Google Scholar]
- 15.Pacilio JE; Tokarski JT; Quiñones R; Iuliucci RJ High-Resoluion Solid-State NMR Spectroscopy: Characterization of Polymorphism in Cimetidine, a Pharmaceutical Compound. J. Chem. Educ., 2014, 91 (8), 1236–1239. [Google Scholar]
- 16.Karwa R; Tran DN; Maina M; Njuguna B; Manji I; Wasike P; Tonui E; Kigen G; Pastakia SD Addressing the 3A’s (Availability, Accountability, Adherence) of Supply Chain Systems in Western Kenya in Mobilizing chemistry expertise to solve humanitarian problems, 2017, ACS Symposium Series; 1267/1268, 129–158. [Google Scholar]
- 17.OSF: Distributed Pharmaceutical Analysis Laboratory (DPAL) https://osf.io/k9ap7/ (accessed 2020–09-09)
- 18.ORCID: Connecting research and researchers. https://orcid.org/ (accessed 2020–09-09)
- 19.Lieberman M What Would You Fight For? Presented at the 25th Biennial Conference on Chemical Education, July 30, 2018. Notre Dame, IN. [Google Scholar]
- 20.Ozawa S; Evans DR; Dessias S; Haynie DG; Yemeke TT; Laing SK; Herrington JE Prevalence and Estimated Economic Burden of Substandard and Falsified Medicines in Low- and Middle-Income Countries: A Systematic Review and Meta-analysis. JAMA Netw Open, 2018, 1(4), e181662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myers NM; Maina MW; Were PM; Karwa R; Pastakia SD; Sharp JC; Luther JL; Cooper A; Bliese SL; Oberhof N; Aldulaimi D; Lieberman M Lab on paper: assay of beta-lactam pharmaceuticals by redox titration. Anal. Met, 2019, 11, 4741–4750. [Google Scholar]
- 22.Bliese SL; Maina MW; Were PM; Lieberman M Detection of degraded, adulterated, and falsified ceftriaxone using paper analytical devices. Anal. Met, 2019, 11, 4727–4732. [Google Scholar]
- 23.Weaver AA; Reiser H; Barstis T; Benvenuti M; Ghosh D; Hunkler M; Joy B; Koenig L; Raddell K; Lieberman M Paper analytical devices for fast field screening of beta lactam antibiotics and anti-tuberculosis pharmaceuticals. Anal. Chem, 2013, 85(13), 6453–6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


