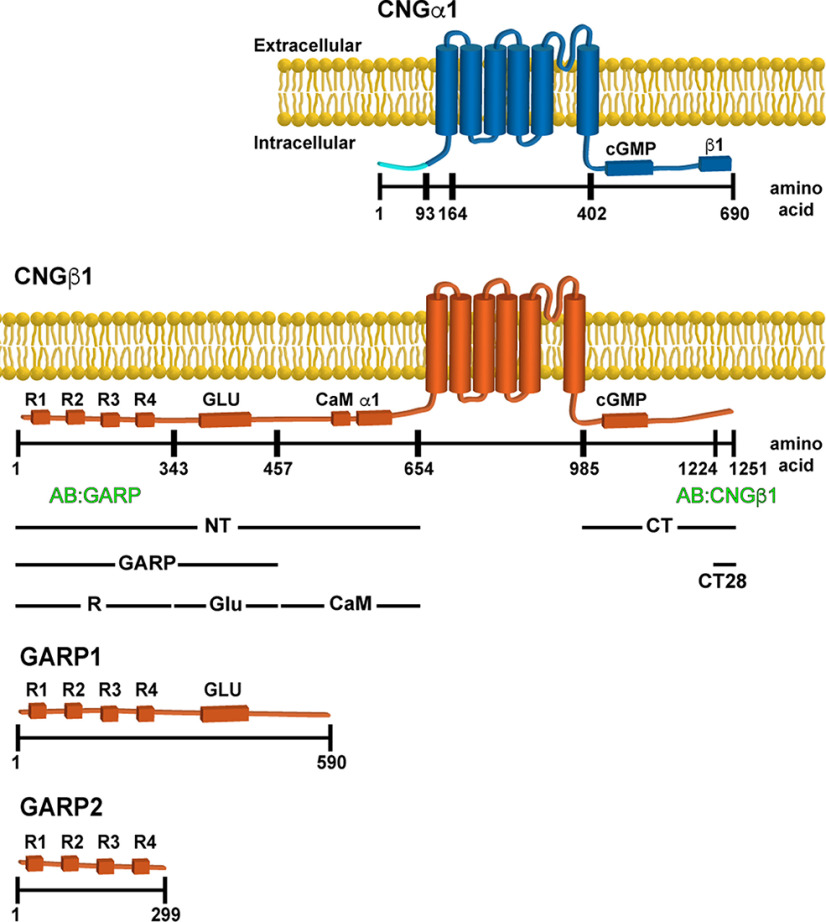
Figure 1.
Domain composition of CNGα1, CNGβ1, GARP1 and GARP2. Cytosolic domains include cGMP binding, calmodulin (CaM) binding, CNGα1 (α1) and CNGβ1 (β1) binding, Glu, and four proline-rich repeats (R1–R4). Notably, the first 92 amino acids of CNGα1 (marked in light blue) are cleaved during intracellular CNG channel processing (Molday et al., 1991). The epitope location for anti-GARP and anti-CNGβ1 antibodies as well as boundaries of CNGβ1-derived constructs expressed in this study are shown below the diagram. The numbering of amino acids corresponds to human protein sequences. For mouse variants, the corresponding amino acids for CNGα1 would be 1, 93, 156, 394, and 684, and for CNGβ1 would be 1, 358, 512, 731, 935, 1278, and 1305.