Figure 7.
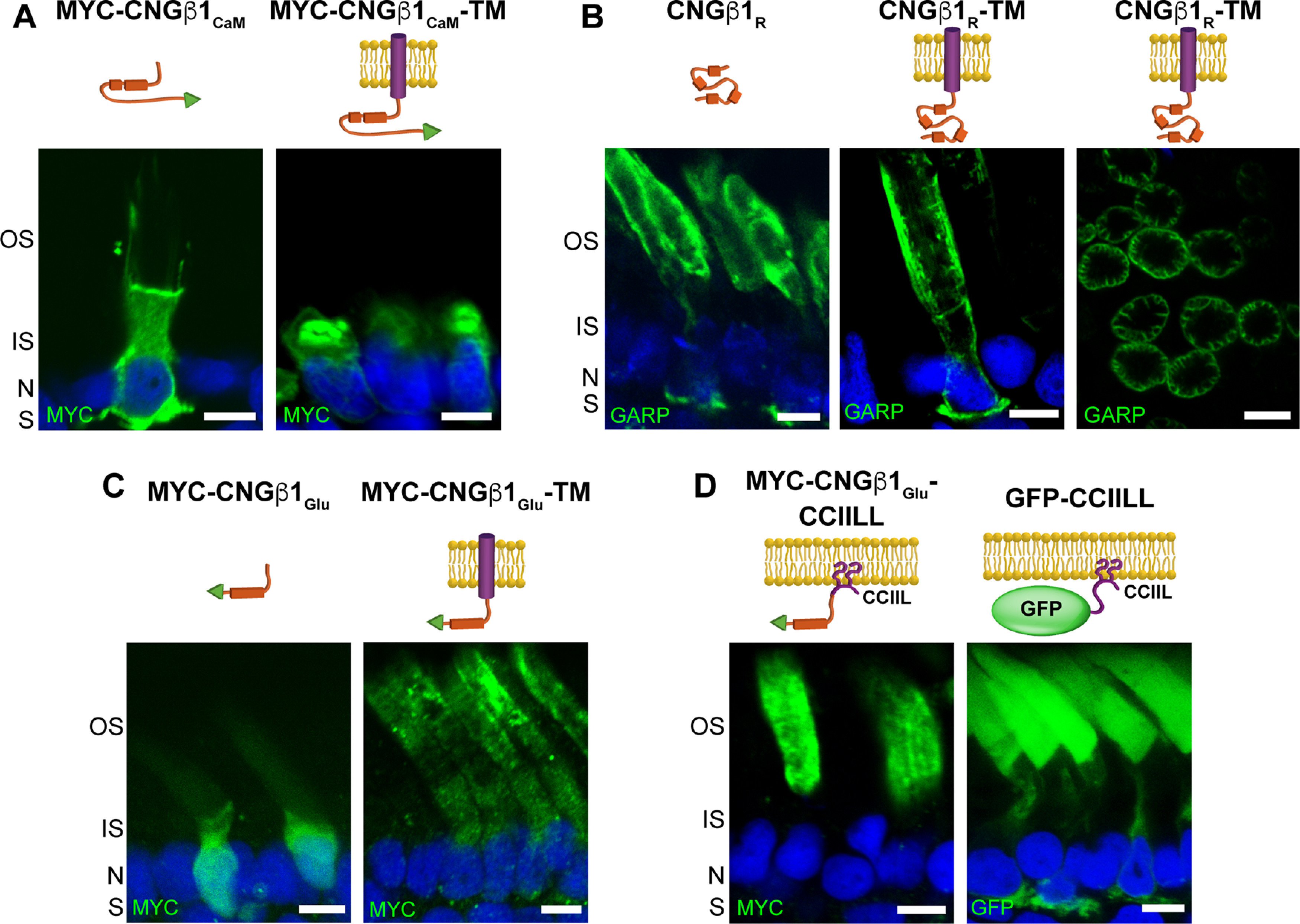
CNGβ1's GARP domain contains separate sites for peripherin-2 binding and outer segment targeting. A–D, Retinal cross-sections from transgenic Xenopus rods comparing localization of soluble and membrane-anchored CNGβ1CaM (A), soluble and membrane-anchored CNGβ1R and a tangential image of outer segment's expressing CNGβ1R-TM (B), soluble and membrane-anchored CNGβ1Glu (C), and CNGβ1Glu or GFP fused to a C-terminal CCIIL double lipidation anchor (D). Cartoon of transgenic constructs are shown above their corresponding panel. Constructs that include the N terminal of human CNGβ1 were detected using an anti-GARP antibody. All other constructs were N-terminally tagged with the MYC epitope and detected using an anti-MYC antibody. Scale bar, 5 µm. Nuclei are counterstained with Hoechst (blue).
