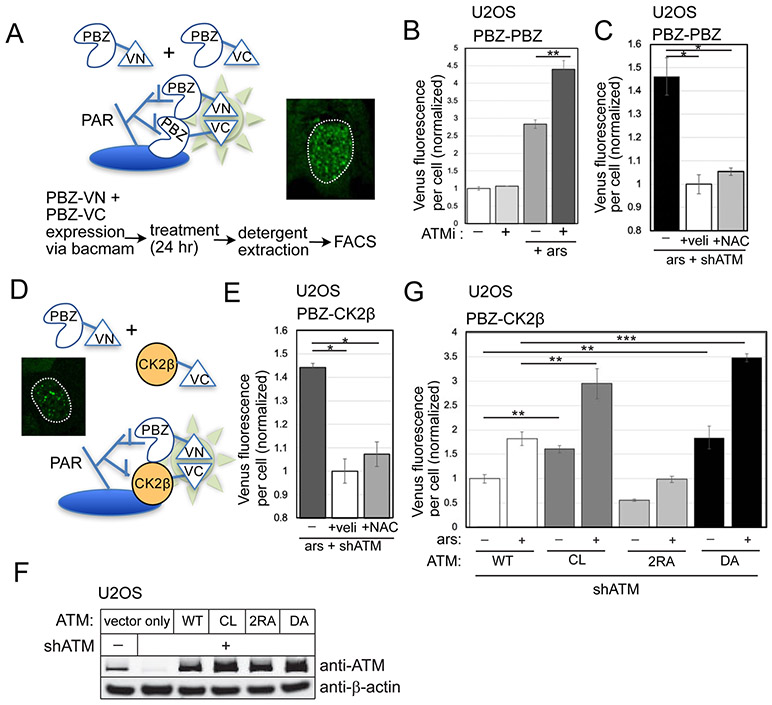
Figure 2. Live-cell sensors for PARylation show higher levels with ATM depletion.
The PBZ-PBZ live-cell split Venus sensor, adapted from (Krastev et al., 2018), with summary of workflow and inset of fluorescence from U2OS cells with ATM inhibitor treatment. (B) FACS results from three replicates showing the mean fluorescence yield per cell from cells expressing the PBZ-PBZ PAR sensor with ATM inhibitor (ATMi, 1 μM AZD1390) and arsenite (25 μM) as indicated. At least 10,000 cells were measured in each replicate; fluorescence yield was normalized to control cells. (C) PBZ-PBZ PAR sensor results with arsenite, ATM shRNA, veliparib (10 μM), and NAC (1 mM) treatment as in (B); fluorescence yield normalized to veliparib-treated cells. (D) Diagram of PBZ-CK2β live-cell split Venus sensor: similar to the PBZ-PBZ sensor but with CK2β replacing the PBZ domain fused to VC, with inset of fluorescence signal from U2OS cells with ATM depletion. (E) FACS results from the PBZ-CK2β sensor with arsenite, ATM shRNA, veliparib, and NAC treatment as indicated; fluorescence yield normalized to veliparib-treated cells. (F) Levels of ATM in U2OS cells with ATM shRNA and expression of recombinant wild-type (WT), C2991L (CL), R2579A/R2580A (2RA), and kinase-deficient D2889A (DA) proteins by western blotting; β-actin shown for normalization. (G) FACS results from the PBZ-CK2β sensor in U2OS cells with depletion of endogenous ATM and expression of recombinant WT, CL, 2RA, or DA alleles, with arsenite as indicated. At least 10,000 cells were measured in each replicate; fluorescence yield normalized to cells expressing WT ATM. Error bars indicate standard deviation. *, **, ***, and **** indicate p<0.05, 0.005, and 0.0005 by Student two-tailed t-test; NS = not significant.