Figure 4. Active transcription and PARylation promote protein aggregation in human cells lacking ATM.
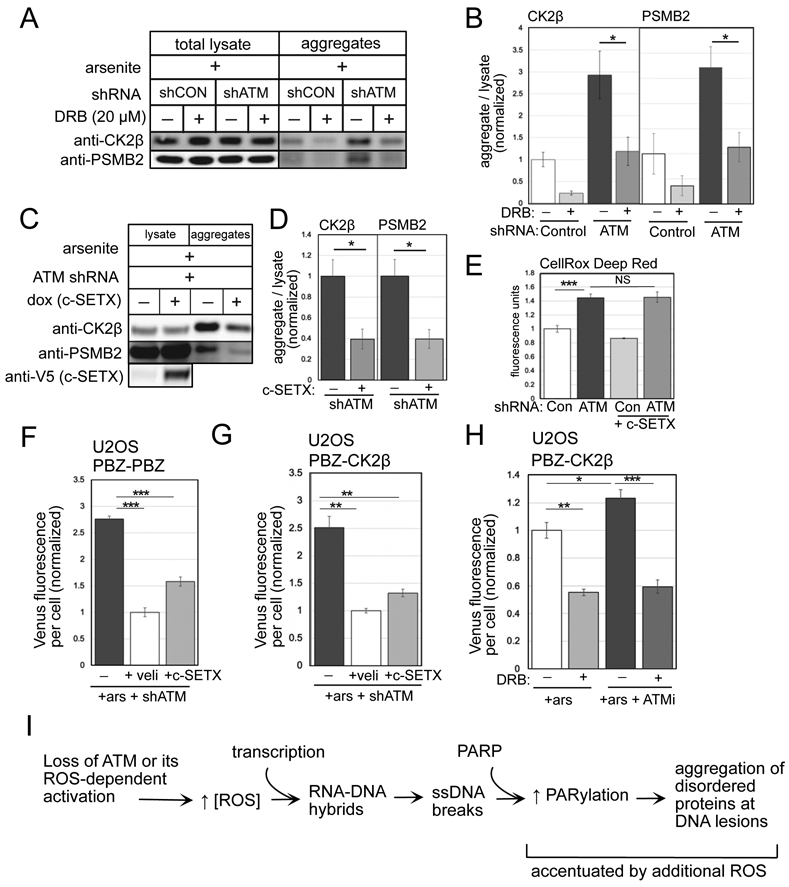
(A) Detergent-resistant aggregates were isolated in U2OS cells with shRNA-mediated depletion of ATM and arsenite (25 μM) and DRB (20 μM) added as indicated. Western blots for CK2β and PSMB2 in lysate and aggregate fractions are shown. (B) Three replicates of (A) were performed and quantified; levels of CK2β and PSMB2 in aggregate fractions normalized by lysate levels are shown relative to control cells. (C) Aggregation assays as in (A) with shRNA depletion of ATM, arsenite, and induction of SETX as indicated. (D) Three replicates of (C) were performed and quantified; levels of CK2β and PSMB2 in aggregate fractions normalized by lysate levels are shown relative to cells without SETX induction. Error (E) Quantification of ROS in U2OS cells with ATM depletion and SETX induction; measured by CellROX in triplicate. (F) Fluorescence yield from the PBZ-PBZ PAR sensor measured in triplicate as in Fig. 2B in ATM-depleted U2OS cells with arsenite and SETX expression as indicated. (G) Fluorescence yield from the PBZ-CK2β sensor measured in triplicate as in Fig. 2E in ATM-depleted U2OS cells exposed to arsenite, and treated with veliparib or induced for SETX expression. (H) Fluorescence yield from the PBZ-CK2β PAR sensor as in Fig. 2E in U2OS cells exposed to arsenite, ATM inhibitor (ATMi, 1 μM AZD1390), and DRB as indicated. Error bars indicate standard deviation. *, **, ***, and **** indicate p<0.05, 0.005, and 0.0005 by Student two-tailed t test; NS = not significant. (I) Diagram of events occurring in ATM-deficient or ATM oxidation activation-deficient cells. An increase in ROS, together with active transcription, generates R-loops and single-strand DNA breaks that hyperactivate PARP, leading to accumulation of disorder-prone proteins at DNA lesions.
