Figure 1.
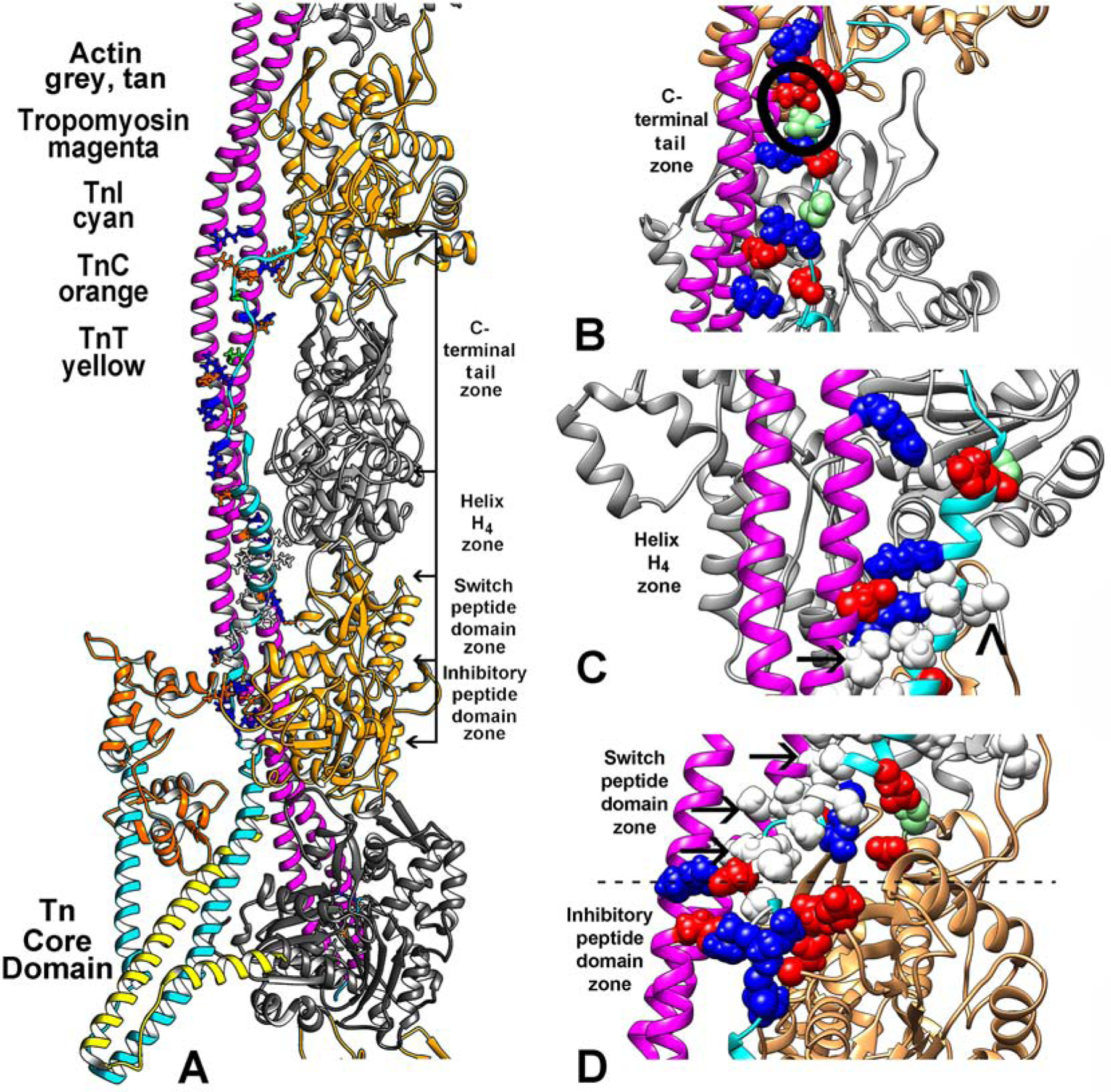
The low-Ca2+ thin filament structure determined in this study. (A) Ribbon diagram focused on the region of the filament over which TnI (cyan) spans actin (grey, tan) and tropomyosin (magenta). The uppermost region of the C-terminal chain of TnI may require zooming to readily locate. The troponin core-domain points (TnI cyan, TnC, orange, TnT, yellow) toward the barbed-end side of actin filament. Only one long-pitch strand of the filament helix is shown for clarity. Selected residues that interface TnI and actin-tropomyosin are highlighted, electrostatic (colored red–acidic, blue-basic), polar (light green) and hydrophobic (white) residues (contacts also noted in (E-G)). (B-D) enlargements of (A) with regions of the filament rotated to focus on segments of TnI at the C-terminal tail end of TnI (B), at its H4–helix region (C), and over the switch-peptide and inhibitory-domains (D). Here, sidechains of interacting resides noted in (A) are drawn in sphere format for emphasis. The inhibitory-peptide seen in (D) makes robust salt-bridge contacts with actin and tropomyosin as first suggested by the Yamada et al. model (see Supplementary Material Fig. S1). In the current study, hydrophobic residues in the TnI switch-peptide and in H4-helix cluster together to abut nonpolar residues on tropomyosin (arrows in (C) indicate examples; a caret points to potential interaction of L369 on TnI and Met47 on the D-loop of actin). In (B), S199, a potential site of TnI phosphorylation, is circled. In addition, salt-bridges link TnI to both actin and tropomyosin here and throughout the structure (see Table 1).
