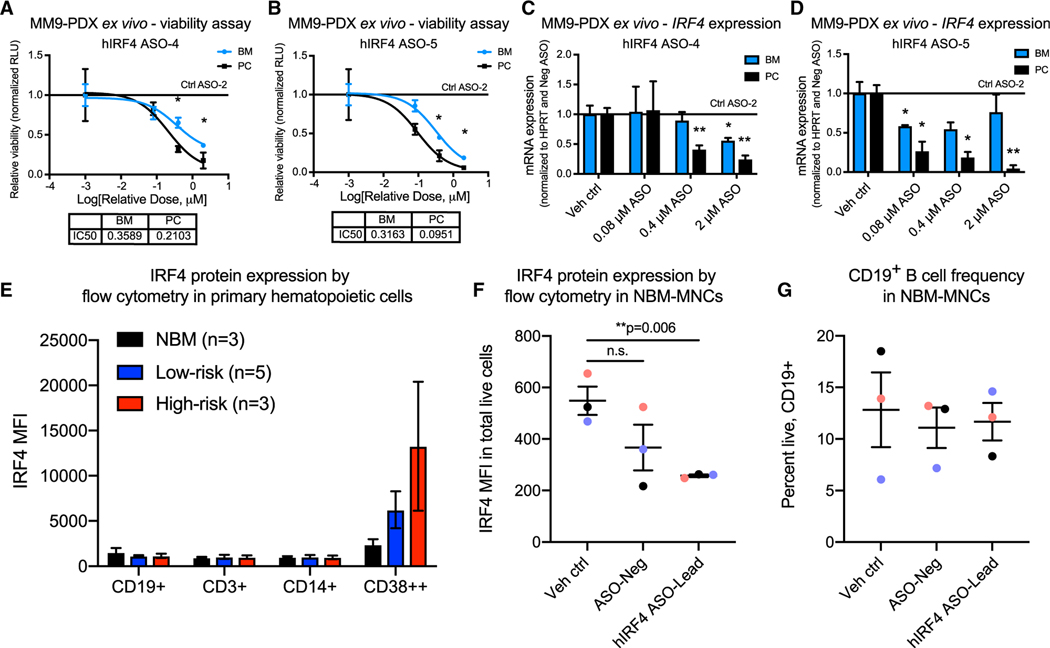
Figure 3. IRF4 inhibition reduces primary patient-derived MM cell viability ex vivo while sparing normal B cell populations.
(A–D) Luminescence-based ex vivo MM9-PDX cell viability assays (A and B) and quantitative RT-PCR analyses (C and D) of BM and PC-derived human cells treated with increasing doses of hIRF4 ASOs, vehicle (PBS) control, or control ASO (up to 2 μM) for 5 days (viability) or 2 days (qPCR; n = 3 individual wells analyzed per tissue for each assay).
(E) Flow cytometry quantification of intranuclear IRF4 expression in primary MNCs from normal bone marrow (NBM) compared with low-risk (smoldering and newly diagnosed MM) and high-risk MM (PCL). MFI was quantified within the live, CD19+, CD3+, CD14+, or CD38++ populations of cells (n = 3–5 samples per group).
(F) Reduced hIRF4 protein expression in NBM samples treated with hIRF4 ASO-Lead (2 μM) compared with PBS control for 3 days.
(G) Unchanged CD19+ B cell frequency after treatment with hIRF4 ASOs as in (F).
Graphs show means ± SEM; *p < 0.05 and **p < 0.01 compared to vehicle or Ctrl ASO-treated cells by unpaired, two-tailed Student’s t test. See also Table S1.