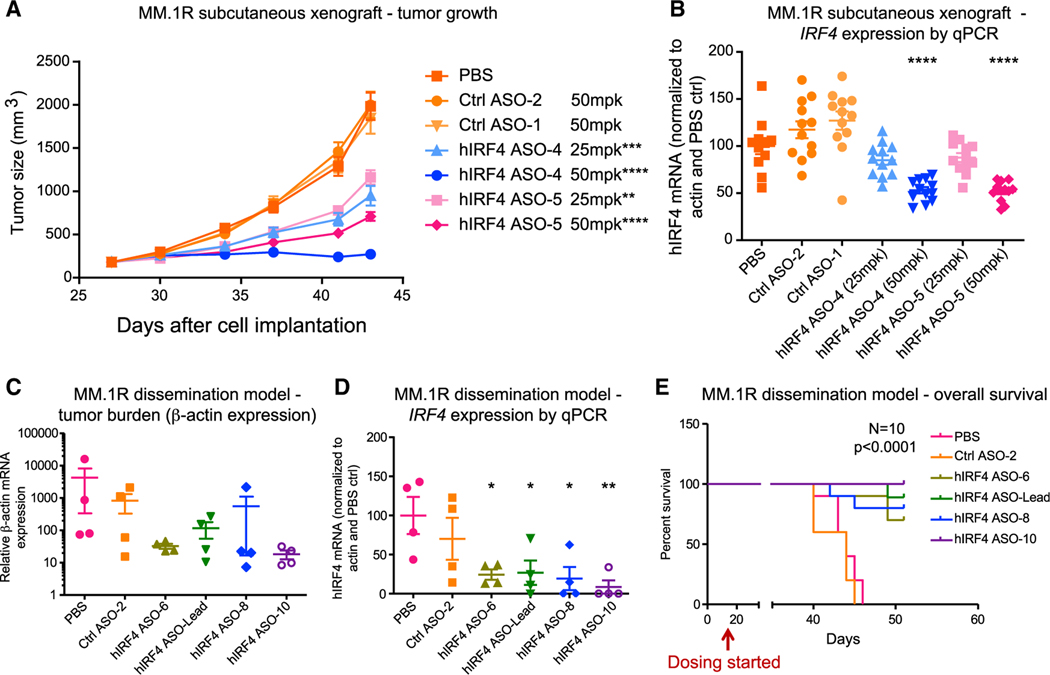
Figure 4. Human IRF4-targeted ASOs reduce tumor burden and IRF4 expression in xenograft models and improve overall survival.
(A and B) For human (h)IRF4 ASO treatment in a subcutaneous xenograft MM model using MM.1R cells, tumor-bearing non-obese diabetic (NOD)/severe combined immunodeficiency (SCID) mice were injected subcutaneously with control (Ctrl) ASOs or IRF4 ASOs (25 or 50 mg/kg [mpk]), or PBS, 5 times per week for 3 weeks (n = 12 mice per group). Average tumor size (A) and quantitative RT-PCR analysis (B) of human IRF4 mRNA expression in tumors from ASO or control mice are shown.
(C–E) In a MM.1R dissemination model, MM cells were transplanted into NSG mice intravenously, followed by treatment with IRF4 ASOs or controls.
(C and D) Tumor burden estimated by qPCR of relative human β-actin levels (C) and human IRF4 mRNA expression (D) in the bone marrow of hIRF4 ASO-treated MM.1R disseminated mice that received three daily doses of ASOs at 50 mg/kg (n = 4 per group).
(E) Overall survival analysis in the MM.1R dissemination model.
Graphs with errors bars show means ± SD; *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 compared to PBS-treated controls by ordinary one-way ANOVA for (A)–(D). Significance was assessed using log rank (Mantel-Cox) test for (E). See also Figure S4.