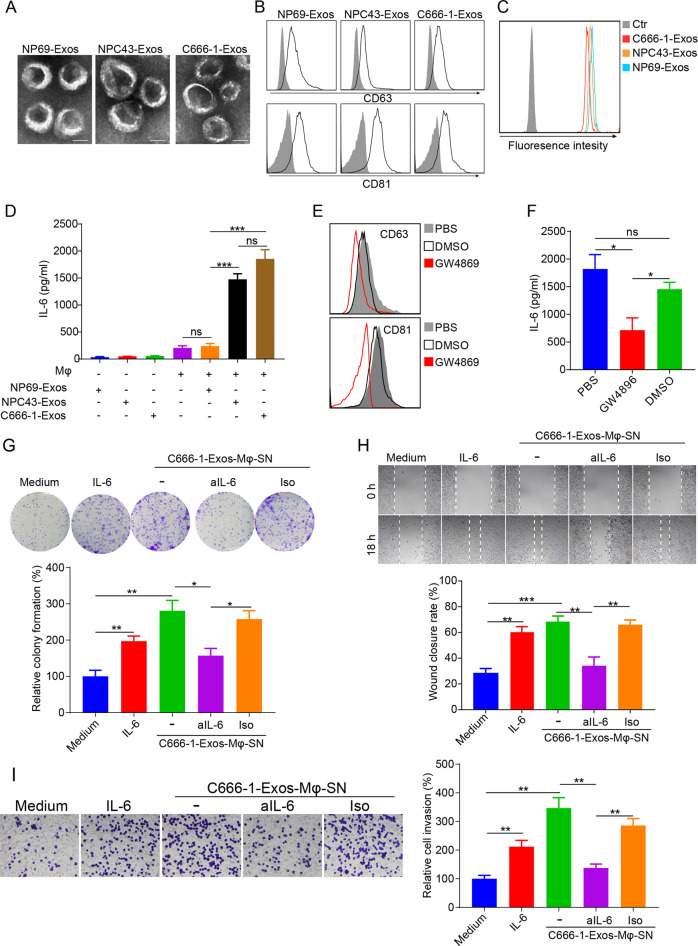
Fig. 1.
NPC-Exos induce IL-6 production from macrophages to promote tumorigenesis. a The morphology of NP69-Exos, NPC43-Exos, and C666-1-Exos was determined by transmission electron microscopy. Scale bar, 50 nm. b Expression of exosomal markers of CD63 and CD81 on exosomes was measured by flow cytometry. c CFSE-labeled exosomes were cultured with THP-1 macrophages for 18 h. The CFSE-positive cells were then detected by flow cytometry. d Expression of IL-6 in exosomes (NP69-Exos, NPC43-Exos, C666-1-Exos) or the supernatant from THP-1 macrophages treated with exosomes for 24 h. e Expression of CD63 and CD81 on ultracentrifuged vesicles isolated from the conditioned culture medium of C666-1 cells pretreated with GW4896, DMSO, or PBS for 6 h. f Expression of IL-6 in the supernatant from THP-1 macrophages treated with ultracentrifuged vesicles isolated from C666-1 cells that had been preincubated with GW4896, DMSO, or PBS for 6 h. The cell proliferation (g), migration (h) and invasion (i) of NPC cells (C666-1) treated with medium, recombinant human IL-6, supernatant from macrophages pretreated with C666-1-Exos, or supernatant from C666-1-Exos-pretreated macrophages plus anti-IL-6 (aIL-6) blocking antibody or its isotype (Iso) were determined by colony formation, wound-healing, and transwell invasion assays. Data are expressed as the means ± SEM and representative of four independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001. NS not significant, Exos exosomes, Mφ macrophages, SN supernatant