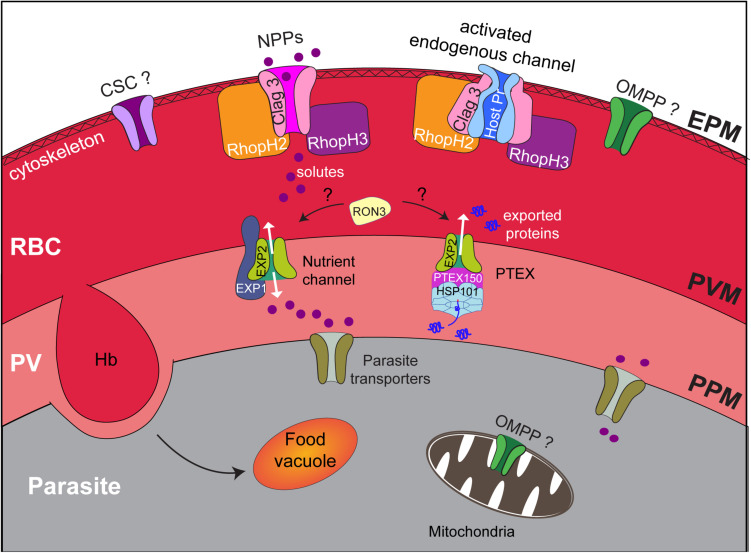
FIGURE 2.
Nutrient acquisition by P. falciparum relies on membrane transporters and the cytostome. Upon infection by Plasmodium parasites, erythrocytes are extensively remodeled to accommodate the growing parasite. The parasite, contained by its own PPM, is encased within a parasitophorous vacuole (PV) and a PV membrane (PVM). Each membrane in the infected erythrocyte, including the erythrocyte plasma membrane (EPM), is modified to include transporters that enable the parasite to access nutrients from its host. These include the NPPs at the EPM, the nutrient channel at the PVM, and transporters at the PPM, whereas hemoglobin uptake and transport to the food vacuole occur via the cytostome. The NPP channel is proposed to be a CLAG3 dimer/oligomer that associates with RhopH2 and RhopH3. Alternatively, CLAG3, RhopH2, and RhopH3 may activate an endogenous host channel. The contribution of other parasite-encoded channels to the NPPs such as the calcium-dependent, stress-gated ion channel (CSC), and OMPP is unknown; the localization of both of these proteins is yet to be ascertained. The nutrient channel at the PVM comprises EXP2, with EXP1 being critical for proper distribution of the EXP2 nutrient channel. EXP2 also forms the pore of the translocon of exported proteins (PTEX). RON3 is implicated in both nutrient uptake across the PVM and protein export with an unknown function. The PPM is also decorated with a raft of transporters that facilitate the uptake of diverse substrates to satisfy the metabolic requirements of the growing parasite.