Figure 1.
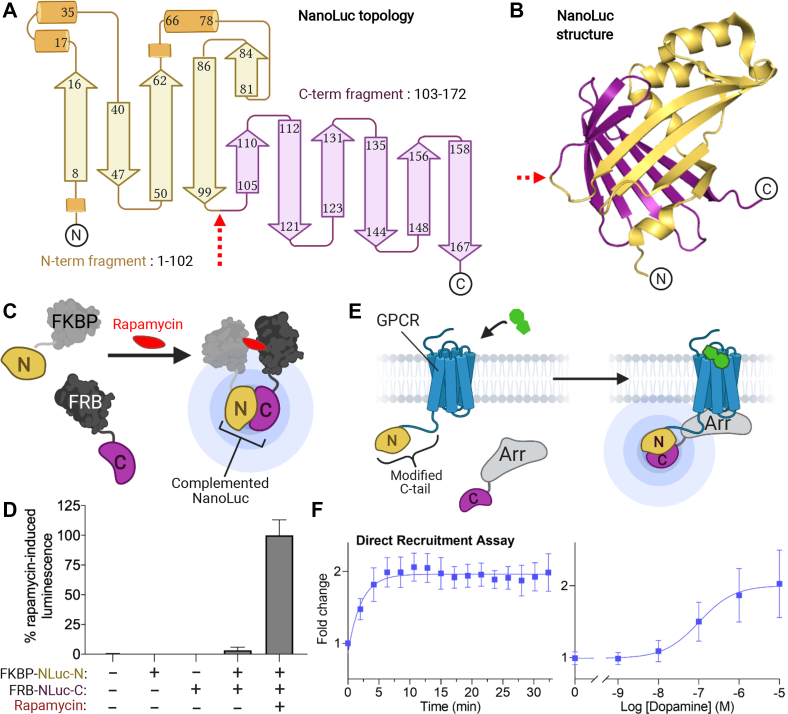
Validation of a novel NanoLuc split for use in complementation-based assays.A, topology of NanoLuc with the unique split site shown with a red arrow and the corresponding N-terminal (residues 1–102) and C-terminal (residues 103–172) split fragments shown in yellow and purple, respectively. The topology diagram is derived from that generated by pro-origami (34). B, structure of NanoLuc (PDB #5IBO) rendered in PyMol (35) showing the N- and C-terminal components in yellow and purple, respectively, and the split site depicted by a red arrow. C, schematic overview of rapamycin-induced dimerization of FKBP and FRB fused to the N- and C-terminal NanoLuc fragments, respectively. D, quantification of luminescence from Hek293 cells in the presence of coelenterazine H for the following conditions: cells with no transfection, cells transiently transfected with constructs coding for either FKBP fused to the N-terminal NanoLuc fragment or FRB fused to the C-terminal NanoLuc fragment alone, and cells transfected with both constructs with and without 50 nM rapamycin treatment. E, schematic describing the direct recruitment assay where the N-terminal and C-terminal NanoLuc fragments are fused to the C terminus of a receptor and to the N terminus of β-arrestin, respectively. F, direct recruitment assay with D2R. Time course after dopamine addition on the left yielded a fold increase in luminescence of 1.96 ± 0.02 that plateaued ∼10 min after 10 μM dopamine treatment and dose–response curve on the right (pEC50: −6.99 ± 0.24). All data represent mean ± SD of three to five independent experiments with triplicate determinations.