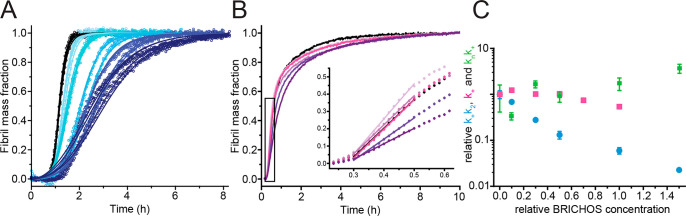
Figure 3.
Aβ42 fibrillation kinetics in the presence of proSP-C BRICHOS
T187R. (A) Individual fits (colored lines) of normalized aggregation
traces of 3 μM Aβ42 alone (black) and in the presence
of 0.1, 0.3, 0.5, 1, and 1.5 molar equivalents of proSP-C BRICHOS
T187R (light blue to dark blue empty circles) using a kinetic nucleation
model in which the combined rate constants 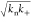 and
and 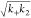 are free fitting parameters. (B) Seeded
reaction profiles of 3 μM Aβ42 with 0.6 μM preformed
fibrils (black) in the presence of 0.1, 0.3, 0.5, 0.7, and 1.0 molar
equivalent of proSP-C BRICHOS T187R (light pink to dark purple). Data
represent the average of four replicates. The black rectangle shows
a close-up of the onset of aggregation. Colored lines are corresponding
fits to the data using a linear regression. (C) Effects of relative
rate constants knk+ (green), k+ (pink), and k+k2 (blue) from
the fits shown in panels A and B. Rates have been normalized to Aβ42
in the absence of BRICHOS. Data represent the mean ± the standard
deviation of four or five replicates.
are free fitting parameters. (B) Seeded
reaction profiles of 3 μM Aβ42 with 0.6 μM preformed
fibrils (black) in the presence of 0.1, 0.3, 0.5, 0.7, and 1.0 molar
equivalent of proSP-C BRICHOS T187R (light pink to dark purple). Data
represent the average of four replicates. The black rectangle shows
a close-up of the onset of aggregation. Colored lines are corresponding
fits to the data using a linear regression. (C) Effects of relative
rate constants knk+ (green), k+ (pink), and k+k2 (blue) from
the fits shown in panels A and B. Rates have been normalized to Aβ42
in the absence of BRICHOS. Data represent the mean ± the standard
deviation of four or five replicates.