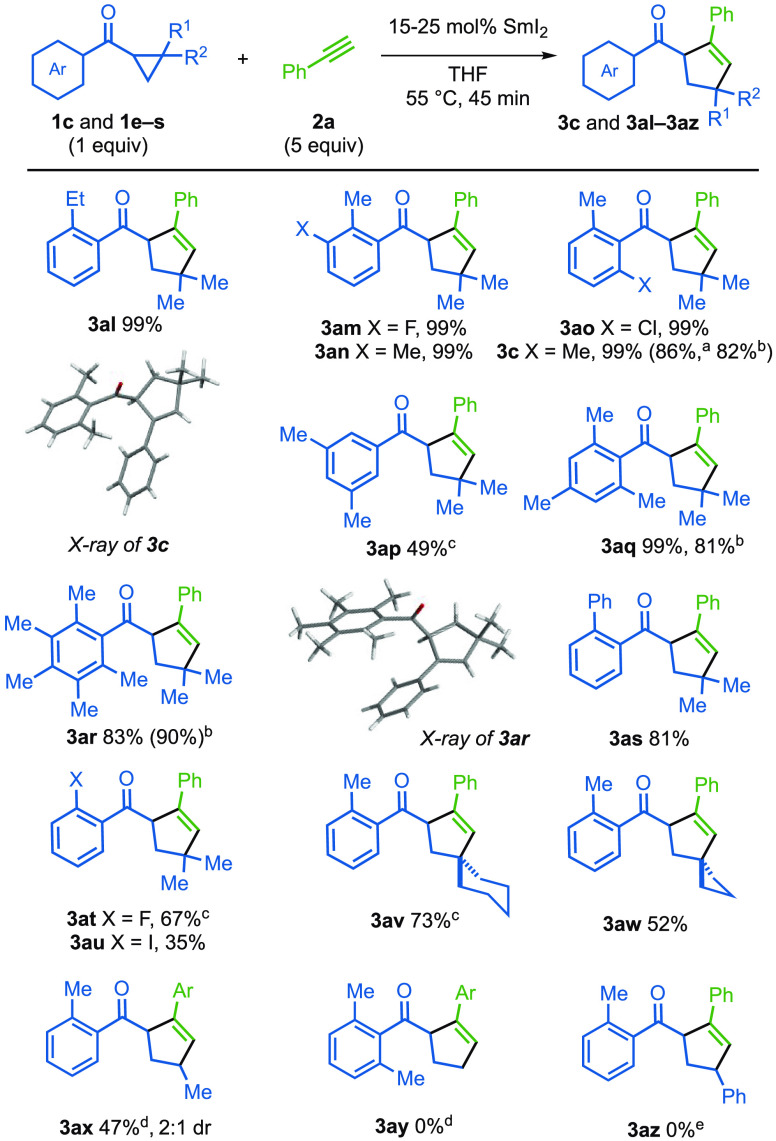
Figure 2.
Scope with respect to the aryl cyclopropyl ketone. Reaction conditions: 1 (1 equiv), 2 (typically 5 equiv), 25 mol % SmI2 (0.1 M in THF), in THF (0.5 mL/0.1 mmol of substrate) under nitrogen. Isolated yields. a Using 1.01 g of ketone partner. b Using 15 mol % of SmI2. c Using 40 mol % SmI2. d 4-Ethynylbenzonitrile was used as the alkyne partner. e Starting ketone was recovered.