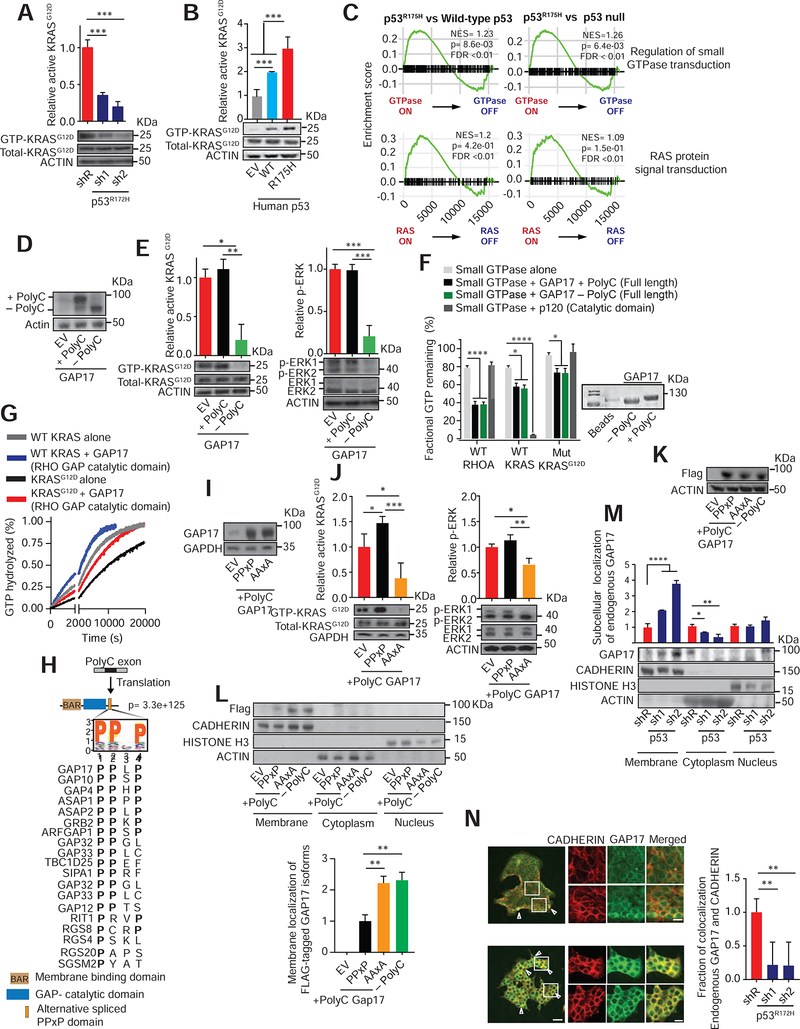
Figure 2: GAP17 isoforms promote GTP-hydrolysis of RAS small-GTPases and impact membrane localization.
A. Affinity precipitation of GTP-bound KRASG12D in KPC cells following transduction with doxycycline-inducible control (shR) or anti-p53 shRNAs (sh1 and 2). Analysis performed after 5 days of doxycycline. Precipitation using GST-Raf1-RBD fusion protein, blotting using RasG12D antibody. Mean ± s.d, n= 3 replicates/condition. Student’s t-test.
B. Affinity precipitation of GTP-bound KRASG12D in murine PDAC cells with null p53 (KPFLC cells) overexpressing human wild-type p53 (WT), p53R175H (R175H) and empty vector (EV). Precipitation and blotting performed as in A. Mean ± s.d, n= 3 replicates/condition. Student’s t-test.
C. GSEA plots showing p53R175H tumors positively associated with small GTPase and RAS-activated gene signatures in PDAC patients (Bailey et al., 2016).
D. Western demonstrating overexpression of + polyC or −polyC GAP17 isoforms or EV in KPC cells.
E. Left, affinity precipitation of GTP-bound active KRASG12D in KPC cells following overexpression of +polyC and −polyC GAP17 isoforms. Precipitation and blotting performed as in A. Right, relative Erk phosphorylation in KPC cells following overexpression of +polyC or −polyC GAP17 isoforms or EV. n = 3 replicates/condition. Mean ± s.d, Student’s t-test.
F. Fractional GTP remaining (%) after combining purified WT KRAS, WT Rho-A, or KRASG12D mutant proteins with purified full-length GAP17 +polyC and −polyC isoform proteins. Catalytic domain of p120 used as control. Measurements taken after 2 h of reaction. Coomassie stain for purified individual full-length +polyC and −polyC GAP17 proteins. Mean ± s.d, n = 3 replicates/condition. Student’s t-test.
G. Real time measurement of GTP hydrolysis of recombinant full-length WT and mutant G12D KRAS and recombinant GAP17 Rho-GAP domain (residues 245–489). Measurements taken up to 20200 sec. Mean ± s.d, n = 3 replicates/condition.
H. Residue motif enrichment encoded by polyC exons in GAPs promoted by p53R175H/−. Bottom, alignment of representative PPXP motifs gained in GAPs by p53R175H/−.
I. Western for expression of GAP17 in KPFLC cells encoding EV or +polyC GAP17 with WT PPXP motif or mutant PPXP motif (“AAxA”).
J. Affinity precipitation of GTP-bound active KRASG12D (left) or p-Erk (right) in KPFLC cells following overexpression of PPXP or AAXA +polyC GAP17 constructs or EV. Precipitation and blotting performed as in A. n = 3 replicates/condition. Mean ± s.d, Student’s t-test.
K. Western for expression of FLAG-tagged GAP17 isoforms (+polyC and −polyC) and +polyC mutant (AAXA) in p53-null murine PDAC KPFLC cells.
L. Relative membrane localization of each FLAG-tagged GAP17 isoform in p53-null PDACs KPFLC cells after 20% FBS stimulation and obtaining cell-membrane enriched fraction. Anti-FLAG antibody used to measure membrane localization. Pan-cadherin: membrane marker; actin: cytoplasmic fraction; histone H3: nuclear fraction. n = 3 replicates/condition. Mean ± s.d, Student’s t-test.
M. Relative membrane and cytosolic localization of endogenous GAP17 in KPC cells following transduction with doxycycline-inducible control (shR) or anti-p53 shRNAs (sh1 and 2). n = 3 replicates/condition. Mean ± s.d, Student’s t-test.
N. Immunofluorescence of endogenous GAP17 in KPC cells following transduction with shR or anti-p53 shRNAs. Arrowheads: membrane co-localization of GAP17 and cadherin. Scale bars: 50 and 5 μm. n = 10–20 random photos/condition. Mean ± s.d, Student’s t-test.
s.d, standard deviation; n, number of repetitions. *p < 0.05, **p < 0.01, ***p < 0.001, **** p < 0.0001. See also Figure S3.