Abstract
Invasion of the brain parenchyma by a meningioma classified by histological criteria as World Health Organization (WHO) grade I meningioma, implies that the tumor has greater likelihood of recurrence and a biological behavior similar to the more aggressive WHO grade II meningiomas. It is therefore important to detect microscopic foci of brain invasion during surgery in order to maximize the resection and/or adapt imaging follow‐up. In this study, we tested the sensitivity of two handheld confocal imaging devices to detect foci of brain invasion in two types of meningioma mouse models: in a genetically engineered mouse model and in a syngeneic xenograft model. Confocal imaging offered precise images of meningothelial and fibroblastic mouse meningiomas as well as malignant meningiomas, which corresponded exactly to the pathological findings. Imaging showed a sharp definition of the brain‐tumor interface and enabled identification of embedded nerves and vessels. Importantly, in both mouse models used in this study, extension of tumor along Virchow–Robin spaces into adjacent brain was detected by imaging. In conclusion, this novel technique, following validation in clinical trials, may open new possibilities for use in operating rooms to influence both decision making during the surgery and planning for additional treatments.
Keywords: brain neoplasm, invasion, meningioma, MGS, mouse model, multimodal confocal imaging
Introduction
Most meningiomas are benign and correspond to World Health Organization (WHO) grade I 11. However, up to 25 % of meningiomas display aggressive histological features correlated with a less favorable clinical outcome and therefore clinically correspond to WHO Grade II (atypical) and WHO grade III (anaplastic) 11, 17. WHO grade II meningiomas present a 28% recurrence rate, occurring within 5 years after resection 1. Brain invasion, occurring either in histologically benign or in atypical tumors, has been correlated with increased recurrence 15. It was therefore decided to consider and treat WHO Grade I meningiomas with brain invasion, as equivalent to WHO grade II tumors 11. Consequently, intraoperative detection and resection of foci of invading meningioma cells is a critical step in improving management and prognosis of these tumors. Although tumor tissue can be macroscopically or microscopically distinguished from normal brain with relative ease in most cases, intraoperative distinction of normal and microscopic foci of infiltrating tumor cells remains challenging.
In this regard, fluorescence‐guided surgery of brain tumors has gained recent attention as it allows to increase the rate of gross total resection in glioblastomas 19. In meningioma surgery, intraoperative 5‐amino‐levulinic‐acid fluorescence has proved to be a useful tool for the visualization of meningioma remnants attached to the brain or infiltrating surrounding bone and dura 2, 7, 13. Indocyanine green fluorescence has also been used in meningioma surgery to ascertain cortical vein patency in the tumor bed after removal of a meningioma and further tumor resection in selected cases 10. However, these techniques remain inadequate for the detection of microscopic invasion of the brain by clusters of meningioma cells.
Optical technologies have recently contributed to the advancement of diagnosis and treatment in some fields of medicine, principally in gastroenterology. Handheld confocal imaging has proved to be a useful tool in the analysis of intact tissue at a cellular level 6. The integration of fiber optics and microscope miniaturization has recently allowed the use of in vivo confocal microscopy in several clinical settings: by incorporating confocal microscopy technology into the distal tip of conventional video endoscopes, cellular imaging of gastrointestinal mucosa was generated, allowing the detection of neoplastic changes 3, 6, 9. The bladder mucosa, skin and cornea have similarly been studied by this techniques 3, 9, 14. In the field of neurosurgery, handheld confocal microscopy has already been tested in xenograft mouse models of glioblastoma to identify the transition zone between tumor and normal tissue and detect invasive tumor cells in the brain parenchyma 5, 12. The value of these studies is nonetheless limited because the use of human tumor cell cultures implanted in the mouse facilitates the recognition of the tumor cells. This technique has also been tested in humans to identify several tumor histological types (including gliomas, meningiomas and one hemangioblastoma) 4 and detect low‐grade glioma cells in the brain parenchyma 18.
In this study, we evaluated if miniaturized handheld confocal microscopy could identify brain invasion by meningioma in a robust meningioma mouse model before initiating human clinical trials. We recently produced a genetically engineered mouse (GEM) model of grade II/III meningioma through neonatal biallelic inactivation of both Nf2 and Cdkn2ab restricted in meningeal cells and developed a grade III mouse meningioma cell line 16. In the GEM model, meningiomas developed from mouse arachnoidal cells at a frequency of 72% after a mean follow‐up of 3.5 months. Of these meningiomas, 66% were grade I, 31% grade II and 3% were grade III tumors. We observed involvement of adjacent brain by meningiomas either by true invasion with direct breaching of the pial surface by meningioma cells or by extension of the tumor along Virchow–Robin spaces. In the syngeneic model using the grade III meningioma cell line, we observed 100% of grade III meningiomas with brain invasion. In this study, we use these new models to test the sensitivity of handheld confocal microscopy in the detection of tumor invasion into brain.
Material and Methods
GEM model of invasive meningioma
In the GEM mouse model of grade II–III meningioma 16, Nf2 and the Cdkn2ab locus (p15Ink4b, p16Ink4a, p19Arf) are inactivated in arachnoidal cells by adenovirus Cre‐mediated excision of Nf2 exon 2 in newborn mice (conditional knockout mouse model). All animal care and experimentation reported herein were conducted in compliance with the guidelines and with the specific approval of Institutional Animal Care and Use Committee of the French Department of Agriculture. The 20 mice included in the study were monitored carefully for signs of brain or spinal cord compression (paraparesis, lethargy) and were sacrificed after 3 months.
Orthotopic syngeneic mouse model of invasive meningioma
In the syngeneic model of invasive grade III meningioma, MGS2 cells derived from a fibroblastic mouse meningioma 16 in adult FVB wild‐type mice were orthotopically injected into the subdural space at the convexity under general anesthesia. A burr hole was performed in the frontal region using a 27G needle after skin incision and 8 μL of the solution (concentration between 1.5 × 104 and 7 × 107 cells/μL) was infused over the course of 1 minute with a 10 μL Hamilton Syringe (Hamilton, Bonaduz, Switzerland), On the 14th day after implantation, the 10 mice included in the study were anesthetized using the xylazine/ketamine mixture as described previously and imaged. At the end of each experiment, anesthetized animals were sacrificed according to our institutional guidelines.
Ex vivo confocal imaging protocol
The devices used for this study were both prototypes (Optiscan Pty. Ltd., Notting Hill, Victoria, Australia) consisting of a handheld rigid probe in which a miniaturized scanner has been integrated and connected via a flexible umbilicus to an optical unit and a control PC unit, which display images in real time. The scanner is based on a scanning technology using a single optical fiber acting as both an illumination and detection pinhole for confocal isolation of the imaging plane. With the visible wavelength fluorescence (VWF) device, excitation by blue laser illumination was at 488 nm wavelength and detection was filtered to green‐yellow light via a 505–585‐nm filter. With the near‐infrared (NIR) device, a 780‐nm diode laser provided incident excitation and fluorescent emission was detected at >795 nm.
VWF imaging protocol
Of the 20 mice analyzed, 16 received intravenous sodium fluorescein (10% in saline, SERB, Paris, France) and all received topical acridine orange hydrochloride (1% in saline, Sigma Chemicals, St. Louis, MO, USA). Fluorescein stains cellular cytoplasms. Acridine orange, by contrast, is taken up intracellularly and stains actively metabolizing cell nuclei. Therefore, the two contrast methods provide complementary imaging detail. Fluorescein was administered as a bolus dose of 200 μL through intraperitoneal injection with a 27‐gauge needle 15 minutes before animal sacrifice. Topical acridine orange was administered as a thin film applied onto the surface of the exposed brain and meningioma using a needle‐less 1 mL syringe and excess dye was washed away with saline.
NIR imaging protocol
Indocyanine green (ICG) is a dye binding almost completely to globulins after intravenous injection. When normal vascular permeability is preserved, it therefore remains in the intravascular compartment. As meningiomas obtained in our GEM model were not highly vascularized, we decided to use the MGS2 cell line to test the ICG imaging device. This malignant mouse meningioma cell line gives rise to 100% of highly vascular brain‐infiltrating tumors when injected orthotopically in FVB mice. All mice received a standard dose of 0.1 mL/g ICG (2.5% in saline, SERB S.A.S, France) administered intravenously into the tail vein immediately after general anesthesia was performed. Skull opening and imaging were performed 15 minutes after injection. When imaging contrast was not sufficient, complementary intratumoral injection was performed.
Image acquisition protocol
In vivo confocal imaging was performed right after animal death. Each mouse was euthanized using carbon dioxide. Mouse skull was opened using scissors. The skull was then gently removed in order to avoid detaching the meningioma from the brain surface. Meningioma was exposed at the brain convexity for the syngeneic model and at the skull base for the GEM model. Meningioma with attached adjacent brain parenchyma was dissected en bloc with the preservation of the cleavage plane between tumor and brain. To summarize the experimental setting, the relationships between the tumor, the brain and the probe during imaging are displayed on an artistic drawing (Figure 1).
Figure 1.
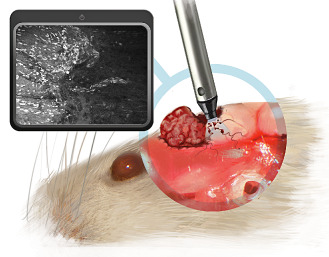
Schematical draft illustrating the relation between mouse brain, tumor and confocal endomicroscopy device in a case of convexity meningioma. The posterior part of the tumor has been removed to scan the tumor bed with the confocal endomicroscopy device and detect invasive meningioma cells along blood vessels in the brain parenchyma.
The probe (Optiscan Pty. Ltd.) was then attached to a handling device (Christoph Hauger, Carl Zeiss, Oberkochen, Germany) and the distal tip lowered gently onto the surface of the specimen. Initial contact was made at the surface of the brain parenchyma. The probe was then moved smoothly to the meningioma. Images were acquired at increasing depths from the surface of the tumor in order to detect the brain‐tumor interface. Images were stored as digital files. Total imaging time with the brain exposed was on the order of 1 h per animal.
Pathological analysis
Once dissected, specimens were immediately marked with tissue ink to indicate superior and inferior surfaces and stored in alcohol‐formalin‐acetic acid (AFA) solution. Specimens were labeled according to mouse number and placed into cassettes. Subsequently, specimens were cut parallel to the brain surface into 2‐μm sections every 50 μm in order to get images in the same plane as the confocal microscopy. Ten slides were obtained for each tumor and stained with hematoxylin and eosin (H&E) for histopathological analysis. Diligent care was taken to ensure correct orientation of the tissue was maintained throughout the handling and staining process so that direct comparisons could be made between confocal microscope images and histological sections from the same areas. In the GEM model, we found 17 meningiomas in 20 mice (85%) among which we observed eight cases of extension along Virchow–Robin spaces (47%). In the syngeneic model, we found 100% of meningiomas with invasion along Virchow–Robin spaces in all cases. There was no relationship between tumor size and the presence of brain infiltration. When comparing the mitotic activity of the invasive part and the mass overlying the brain in our tumors, we observed that the tumor bulk and the perivascular tumor cells had similar mitotic activity (Figure 2). The examples displayed demonstrate that there is no difference in proliferation rate between the noninvasive and invasive parts of a single meningioma.
Figure 2.
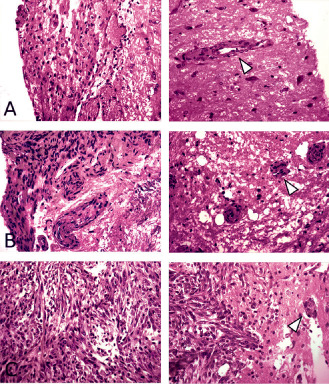
Comparison of mitotic activity in the meningioma bulk and the invasive meningioma cells. For each of the three cases, the meningioma is displayed on the left and the invasive part on the right. White arrow heads: clusters of meningioma cells around blood vessels in the brain parenchyma. In cases A and B, meningioma and perivascular invasion pairs show similar mitotic activity—no mitosis was identified in either part. On the other hand, case C shows multiple mitotic figures in both components—the tumor mass overlying the brain and the invasive perivascular (and intraparenchymal) cells.
Results
Confocal microscopy reproduces the histopathological features of meningothelial, fibroblastic and anaplastic meningiomas and identified the brain‐meningioma interface
Over the 17 meningiomas found on pathological analysis in the GEM model, 15 were located at the skull base and two at the convexity. Most meningiomas were meningothelial (n = 11) but there were also tumors of transitional (n = 5) and fibroblastic (n = 1) histological subtype. We first tested the ability of confocal imaging to differentiate meningothelial and fibroblastic variants of meningioma.
Confocal images of meningothelial and fibroblastic meningiomas reproduced the histological patterns of their histological subtype (Figure 3A,B). The confocal images of uniform oval nuclei set on a plain background in the meningothelial variant corresponded well to the lobules of tumor cells that appear to form a syncytium observed on H&E‐stained sections (Figure 3A). Conversely confocal images with retractile fibers intermingled with elongated nuclei corresponded well to collagen bundles observed on H&E‐stained sections in fibroblastic tumors (Figure 3B). Figure 3C,D demonstrates two examples of histological sections of meningioma and adjacent brain tissue. Confocal imaging illustrated the specific features typical of these tissues as seen on pathology: tumor tissue demonstrated dense cellularity and a high cytoplasmic fluorescence, which contrasts with the low cellularity and low cytoplasmic fluorescence of the normal cortex. The brain/tumor interface was sharp in both cases.
Figure 3.
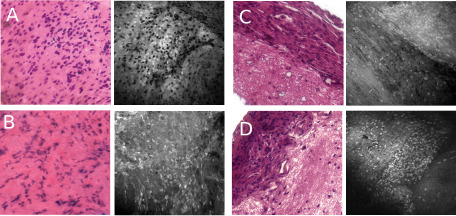
Confocal imaging of meningothelial and fibroblastic mouse meningiomas. For each image, H& E‐stained section is displayed on the left panel and confocal image is shown on the right panel. A. Meningothelial meningioma imaged with fluorescein only. B. Fibroblastic meningioma. C,D. Imaging of the tumor border demonstrating the sharp transition between cellular meningothelial tissue and brain parenchyma.
In the syngeneic model, we obtained grade III meningiomas characterized by architectural dedifferentiation in all implanted mice. Confocal microscopic imaging allowed identification of different tumor regions including areas with high cellular density and high nucleocytoplasmic index (Figure 4A), low cellular density (Figure 4B) and necrosis (Figure 4C) all of which correlated well to pathological findings.
Figure 4.
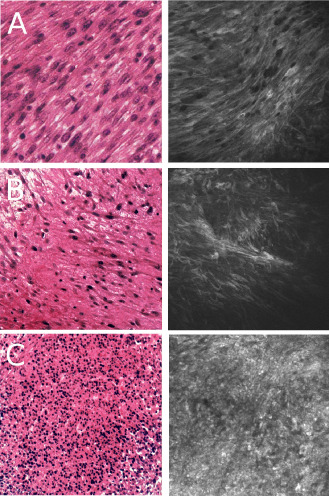
Confocal imaging of malignant mouse meningiomas. For each image, H& E‐stained section is displayed on the left panel and confocal image is shown on the right panel. Imaging of grade III mouse meningioma syngeneic xenografts. A. Cellular imaging strikingly reproduces the sarcoma‐like appearance of grade III meningiomas. B. Imaging of decreased cellular density regions also shows substantial resemblance with histopathology. C. Intratumoral necrosis.
Confocal microscopy allows identification of intratumoral vessels and nerves
Meningiomas at times encase cranial nerves and normal blood vessels, which are often difficult to locate inside the tumor during surgery. Consequently, we tried to determine if handheld confocal imaging could help distinguish these structures within the tumor bulk. As meningiomas mostly develop at the ventral side of the brainstem in our GEM model, they often encompass cranial nerves and branches of the vertebral or basilar arteries. Figure 5A demonstrates a normal vessel seen below the tumor surface. Cellular imaging convincingly distinguished the tumor lumen within the meningioma but also reproduces the histological features of endothelial border cells. Handheld confocal imaging also reproduces the shape of axons encased inside the tumor bulk as seen on Figure 5B where a nerve is seen next to the meningioma cells and the brain parenchyma, as observed on corresponding pathological section.
Figure 5.
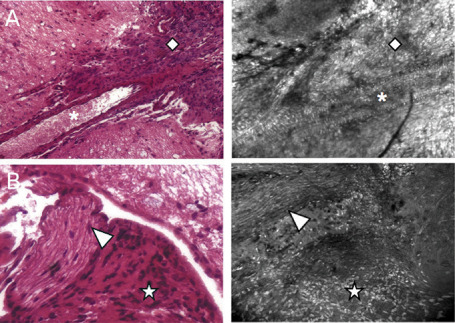
Identification of intratumoral nerves and vessels through handheld confocal imaging. For each image, H& E‐stained section is displayed on the left panel and confocal image is shown on the right panel. Detection of intratumoral nerves (A) and vessels (B) by handheld confocal imaging. Losange: meningioma; asterisk: blood vessel lumen; arrow head: nerve; star: meningioma.
Handheld confocal microscopy identifies regions of brain invasion along Virchow–Robin spaces
In the GEM model, we were able to detect tumor extension along Virchow–Robin spaces using handheld confocal microscopy, as demonstrated on Figure 6A. Cellular imaging clearly identifies meningothelial cells inside the brain parenchyma because of their specific shape and perivascular distribution. This result correlated well to the findings of the pathology analyses. Among the eight cases of meningiomas invading along Virchow–Robin spaces on pathological analysis, a unquestionable correlation to confocal images was confirmed in only one case.
Figure 6.
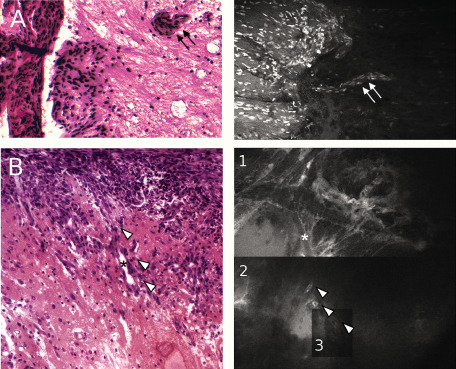
Confocal imaging of brain invasion. A. Hematoxylin eosin safran (HES) slide (left panel) demonstrating invasion of meningothelial cells from the meningioma inside the brain parenchyma along blood vessels (double black arrow). Confocal image (right panel) reliably reproduces the image of Virchow–Robin spaces invasion (double white arrow). B. HES slide (left panel) illustrates the pattern of brain invasion found in the orthotopic syngeneic model (OSM) model. Confocal image (right panel) is divided in three parts (noted 1, 2 and 3) corresponding of three different levels of depth at the same location. The vessel lumen (asterisk) going inside the brain can be clearly identified while scattered tumor cells (white arrowheads) are found at each level of depth.
In the syngeneic model, we were also able to clearly follow tumor cells inside the brain parenchyma along vessels in one case, as displayed on Figure 6B. Although all meningiomas demonstrated extension along Virchow–Robin spaces, there was no other image of brain invasion seen on confocal imaging.
Discussion
This study demonstrates the usefulness of handheld confocal microscopy in the detection of brain invasion by meningioma in a relevant mouse model of meningioma. Similarly, handheld confocal imaging allows identification of tumor margins, differentiates the histological subtypes of meningioma, and identifies encased nerves and vessels within the tumor mass.
Handheld confocal imaging could then be successfully used in the clinical practice to detect microscopic foci of brain invasion by meningiomas. This would allow a more precise classification of such tumors that would be then treated and followed as grade II meningiomas, without the need for hazardous biopsies of adjacent brain parenchyma.
Despite promising applications, handheld confocal imaging devices still present some limitations. As images obtained with confocal imaging are orthogonal to the imaging plane, it may be difficult to detect small regions of brain invasion, more easily spotted on sections tangential to the tumor surface. It is therefore not surprising that we were not able to detect the invasion by breaching of the pial surface both on pathological sections and confocal images. In contrast, tumor extension into Virchow–Robin spaces occurring along brain vessels that are mostly tangential to the brain surface was easier to detect with handheld confocal imaging with nevertheless false‐negative cases. Developing a 3D reconstruction of images acquired with handheld confocal imaging could help obtain a section plane that is more easily readable during surgery. Moreover, the size of the probe tip was large compared to the size of the tumors and of the brain‐meningioma interface, making it difficult to image precisely. This discrepancy could explain the low rate of detection of brain extension along Virchow–Robin spaces by cellular imaging in regard of pathological findings in both models. It is nonetheless linked to the limitations of mouse studies and should not occur in future human trials. Our study demonstrates that both NIR and VWF devices are suitable to detect meningioma invasion. Nonetheless, acridine orange is not approved for use in a clinical neurosurgical setting because it is mutagen and ICG might not be relevant to image meningioma tissue as the first study using ICG during meningioma surgery has not specified its diffusion into the tumor 10. Therefore, we consider intravenous fluorescein more suitable for fluorescence‐guided meningioma surgery up to now.
Apart from detecting invasive meningioma cells, handheld confocal imaging can have other applications in the field of meningioma surgery. As residual clusters of tumor cells found in thick arachnoid membranes of the tumor bed may also be responsible for recurrence 8, resecting those membranes remains a matter of debate as aggressive resection can lead to cortical injury and a risk of seizures. In this scenario, handheld confocal microscopy could also be helpful for the detection of such meningioma cells. Finally, in skull base surgery, confocal imaging could help distinguish elongated nerves at the surface of the tumor when not amenable to neurostimulation.
Even if handheld confocal imaging could help identify aggressive histological features of meningioma during surgery, we do not believe it should aim at replacing frozen sections, which are more reliable and rarely influence the surgical strategy in this particular tumor type when tumors can be totally removed.
The definitive impact on resection and survival of handheld confocal imaging should therefore be tested in an appropriate clinical trial focusing on recurrent and/or high‐grade meningiomas with preoperative brain edema in non‐eloquent regions to get confocal images where brain invasion is suspect. As regions of brain invasion are often focal and associated with preoperative brain edema on magnetic resonance imaging (MRI), scanning only the selected most suspicious regions of the tumor bed with the confocal endomicroscope should not be too time consuming. Fluorescence‐guided surgery could also help identify these regions of suspected brain invasion. As macroscopic fluorescence‐guided surgery and handheld confocal microscopy may both work with identical fluorophores, the combination of these two techniques could prove cost‐effective while allowing an extensive exploration of both dura mater, calvaria and brain parenchyma.
In conclusion, we have demonstrated in this study the capacity of handheld confocal microscopy to detect brain invasion by meningioma cells in a robust meningioma mouse model that mimics perfectly the human pathology, opening the way to future studies to assess its potential clinical application.
Conflict of Interest
The authors have reported no conflicts of interest.
Acknowledgments
The confocal endomicroscope systems were provided by Carl Zeiss Surgical, Gmbh, which also provided engineering and operational assistance. The authors listed on this paper have no financial or marketing relationship with Carl Zeiss Surgical, Gmbh. We would like to thank D. Briant for his assistance to complete the illustration in Figure 1.
References
- 1. Aghi MK, Carter BS, Cosgrove GR, Ojemann RG, Amin‐Hanjani S, Martuza RL et al (2009) Long‐term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery 64:56–60. [DOI] [PubMed] [Google Scholar]
- 2. Coluccia D, Fandino J, Fujioka M, Cordovi S, Muroi C, Landolt H (2010) Intraoperative 5‐aminolevulinic‐acid‐induced fluorescence in meningiomas. Acta Neurochir (Wien) 152:1711–1719. [DOI] [PubMed] [Google Scholar]
- 3. Dunbar KB, Canto MI (2010) Confocal laser endomicroscopy in Barrett's esophagus and endoscopically inapparent Barrett's neoplasia: a prospective, randomized, double‐blind, controlled, crossover trial. Gastrointest Endosc 72:668. [DOI] [PubMed] [Google Scholar]
- 4. Eschbacher J, Martirosyan NL, Nakaji P, Sanai N, Preul MC, Smith KA et al (2012) In vivo intraoperative confocal microscopy for real‐time histopathological imaging of brain tumors. J Neurosurg 116:854–860. [DOI] [PubMed] [Google Scholar]
- 5. Foersch S, Heimann A, Ayyad A, Spoden GA, Florin L, Mpoukouvalas K et al (2012) Confocal laser endomicroscopy for diagnosis and histomorphologic imaging of brain tumors in vivo. Plos ONE 7:e41760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goetz M, Memadathil B, Biesterfeld S, Schneider C, Gregor S, Galle PR et al (2007) In vivo subsurface morphological and functional cellular and subcellular imaging of the gastrointestinal tract with confocal mini‐microscopy. World J Gastroenterol 13:2160–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kajimoto Y, Kuroiwa T, Miyatake S, Ichioka T, Miyashita M, Tanaka H, Tsuji M (2007) Use of 5‐aminolevulinic acid in fluorescence‐guided resection of meningioma with high risk of recurrence. Case report. J Neurosurg 106:1070–1074. [DOI] [PubMed] [Google Scholar]
- 8. Kamitani H, Masuzawa H, Kanazawa I, Kubo T (2001) Recurrence of convexity meningiomas: tumor cells in the arachnoid membrane. Surg Neurol 56:228–235. [DOI] [PubMed] [Google Scholar]
- 9. Kiesslich R, Gossner L, Goetz M, Dahlmann A, Vieth M, Stolte M et al (2006) In vivo histology of Barrett's esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol 4:979–987. [DOI] [PubMed] [Google Scholar]
- 10. Kim EH, Cho JM, Chang JH, Kim SH, Lee KS (2011) Application of intraoperative indocyanine green videoangiography to brain tumor surgery. Acta Neurochir (Wien) 153:1487–1495. discussion 94–95. [DOI] [PubMed] [Google Scholar]
- 11. Louis D, Ohgaki H, Wiestler O, Cavenee W (2007) WHO Classification of Tumours of the Central Nervous System, 4th edn. IRAC Press: Lyon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martirosyan NL, Cavalcanti DD, Eschbacher JM, Delaney PM, Scheck AC, Abdelwahab MG et al (2011) Use of in vivo near‐infrared laser confocal endomicroscopy with indocyanine green to detect the boundary of infiltrative tumor. J Neurosurg 115:1131–1138. [DOI] [PubMed] [Google Scholar]
- 13. Morofuji Y, Matsuo T, Hayashi Y, Suyama K, Nagata I (2008) Usefulness of intraoperative photodynamic diagnosis using 5‐aminolevulinic acid for meningiomas with cranial invasion: technical case report. Neurosurgery 62(3 Suppl. 1):102–103. discussion 3–4. [DOI] [PubMed] [Google Scholar]
- 14. Neumann H, Vieth M, Raithel M, Mudter J, Kiesslich R, Neurath MF (2010) Confocal laser endomicroscopy for the in vivo detection of intraepithelial neoplasia in Peutz‐Jeghers polyps. Endoscopy 42(Suppl. 2):E139–E140. [DOI] [PubMed] [Google Scholar]
- 15. Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM (1997) Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol 21:1455–1465. [DOI] [PubMed] [Google Scholar]
- 16. Peyre M, Stemmer‐Rachamimov A, Clermont‐Taranchon E, Quentin S, El‐Taraya N, Walczak C et al (2012) Meningioma progression in mice triggered by Nf2 and Cdkn2ab inactivation. Oncogene [Epub ahead of print; doi: 10.1038/onc.2012.436]. [DOI] [PubMed] [Google Scholar]
- 17. Riemenschneider MJ, Perry A, Reifenberger G (2006) Histological classification and molecular genetics of meningiomas. Lancet Neurol 5:1045–1054. [DOI] [PubMed] [Google Scholar]
- 18. Sanai N, Snyder LA, Honea NJ, Coons SW, Eschbacher JM, Smith KA, Spetzler RF (2011) Intraoperative confocal microscopy in the visualization of 5‐aminolevulinic acid fluorescence in low‐grade gliomas. J Neurosurg 115:740–748. [DOI] [PubMed] [Google Scholar]
- 19. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ (2006) Fluorescence‐guided surgery with 5‐aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401. [DOI] [PubMed] [Google Scholar]


